Open Thesis.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bioactive Compounds in Nuts and Edible Seeds: Focusing on Brazil Nuts and Baru Almond of the Amazon and Cerrado Brazilian Biomes
Review Article SM Journal of Bioactive Compounds in Nuts and Nutrition and Edible Seeds: Focusing on Brazil Nuts Metabolism and Baru Almond of the Amazon and Cerrado Brazilian Biomes Egea MB1*, Lima DS1, Lodete AR1 and Takeuchi K1,2* 1Science and Technology, Goiano Institute of Education, Brazil 2Faculty of Nutrition, Federal University of Mato Grosso, Brazil Article Information Abstract Received date: Oct 09, 2017 The biodiversity of the Amazon and Cerrado biomes is extremely important for the populations that inhabit Accepted date: Nov 14, 2017 these areas, through the extractive collection of non-timber forest products such as fruits, nuts and edible seeds, which generate income and employment. Brazil nut (Bertholletia excelsa) is native from South America being Published date: Nov 20, 2017 found in the Amazon biome and baru almond (Dipteryx alata Vog.) is native from the Cerrado biome; these are part of the group of oleaginous that can be classified as true nuts and edible seeds, respectively. Both *Corresponding author are important sources of micronutrients that have been associated with several benefits to human health due to the presence of high levels of biologically active compounds such as minerals and vitamins. Minerals act Egea MB, Science and Technology, mostly as cofactors in various reactions, selenium has high availability in Brazil nuts and from selenocysteine Goiano Institute of Education, Brazil, and its enzymes, it exerts functions in the human body as an antioxidant, regulator of thyroid hormones and Tel: +55 64 36205636; protection of cardiovascular diseases. Among vitamins, tocopherol is a precursor to vitamin E, present in both Brazil nut and baru almond, being found in the form of α-tocopherol and having a role in the prevention of various Email: [email protected] diseases, including: cancer, diabetes, cataracts and cardiovascular and cerebrovascular diseases. -
![Hyphaene Petersiana Klotzsch Ex Mart. [ 1362 ]](https://docslib.b-cdn.net/cover/3448/hyphaene-petersiana-klotzsch-ex-mart-1362-313448.webp)
Hyphaene Petersiana Klotzsch Ex Mart. [ 1362 ]
This report was generated from the SEPASAL database ( www.kew.org/ceb/sepasal ) in August 2007. This database is freely available to members of the public. SEPASAL is a database and enquiry service about useful "wild" and semi-domesticated plants of tropical and subtropical drylands, developed and maintained at the Royal Botanic Gardens, Kew. "Useful" includes plants which humans eat, use as medicine, feed to animals, make things from, use as fuel, and many other uses. Since 2004, there has been a Namibian SEPASAL team, based at the National Botanical Research Institute of the Ministry of Agriculture which has been updating the information on Namibian species from Namibian and southern African literature and unpublished sources. By August 2007, over 700 Namibian species had been updated. Work on updating species information, and adding new species to the database, is ongoing. It may be worth visiting the web site and querying the database to obtain the latest information for this species. Internet SEPASAL New query Edit query View query results Display help In names list include: synonyms vernacular names and display: 10 names per page Your query found 1 taxon Hyphaene petersiana Klotzsch ex Mart. [ 1362 ] Family: PALMAE Synonyms Hyphaene benguellensis Welw. Hyphaene benguellensis Welw. var. plagiocarpa (Dammer)Furtado Hyphaene benguellensis Welw. var. ventricosa (Kirk)Furtado Hyphaene ventricosa J.Kirk Vernacular names (East Africa) [nuts] dum [ 2357 ] (Zimbabwe) murara [ 3023 ], ilala [ 3030 ] Afrikaans (Namibia) makalanie-palm [ 5083 -

Fats Ebook Feb 02.Pdf
2 DRHYMAN.COM Contents Contents INTRODUCTION ................................. 8 PART I ........................................... 11 Dietary Fats: The Good, Bad and the Ugly ............................................ 11 Fatty Acids ............................................................................................ 11 Saturated Fat ........................................................................................ 12 Polyunsaturated Fats ............................................................................ 14 Essential Fatty Acids 101- Omega-3 and Omega-6 ............................... 14 The Beneficial Omega-6 Fatty Acid: GLA ............................................... 16 How Fatty Acids Affect Brain Health ..................................................... 17 Omega-7 Fatty Acids ............................................................................ 18 Monounsaturated Fat ............................................................................ 18 Trans Fats ............................................................................................. 20 Trans Fats and Health ........................................................................... 21 Toxins in Fat .......................................................................................... 22 A Case for Organic ................................................................................ 23 DRHYMAN.COM 3 PART II .......................................... 24 Animal Fats ....................................................................... -

Edible Seeds
List of edible seeds This list of edible seeds includes seeds that are directly 1 Cereals foodstuffs, rather than yielding derived products. See also: Category:Cereals True cereals are the seeds of certain species of grass. Quinoa, a pseudocereal Maize A variety of species can provide edible seeds. Of the six major plant parts, seeds are the dominant source of human calories and protein.[1] The other five major plant parts are roots, stems, leaves, flowers, and fruits. Most ed- ible seeds are angiosperms, but a few are gymnosperms. The most important global seed food source, by weight, is cereals, followed by legumes, and nuts.[2] The list is divided into the following categories: • Cereals (or grains) are grass-like crops that are har- vested for their dry seeds. These seeds are often ground to make flour. Cereals provide almost half of all calories consumed in the world.[3] Botanically, true cereals are members of the Poaceae, the true grass family. A mixture of rices, including brown, white, red indica and wild rice (Zizania species) • Pseudocereals are cereal crops that are not Maize, wheat, and rice account for about half of the grasses. calories consumed by people every year.[3] Grains can be ground into flour for bread, cake, noodles, and other • Legumes including beans and other protein-rich food products. They can also be boiled or steamed, ei- soft seeds. ther whole or ground, and eaten as is. Many cereals are present or past staple foods, providing a large fraction of the calories in the places that they are eaten. -

Working Paper Series
Working Paper No. 58, 2013 Landscapes of Freedom and Inequality Environmental Histories of the Pacific and Caribbean Coasts of Colombia Claudia Leal and Shawn Van Ausdal Working Paper Series desiguALdades.net Research Network on Interdependent Inequalities in Latin America desiguALdades.net Working Paper Series Published by desiguALdades.net International Research Network on Interdependent Inequalities in Latin America The desiguALdades.net Working Paper Series serves to disseminate first results of ongoing research projects in order to encourage the exchange of ideas and academic debate. Inclusion of a paper in the desiguALdades.net Working Paper Series does not constitute publication and should not limit publication in any other venue. Copyright remains with the authors. Copyright for this edition: Claudia Leal and Shawn Van Ausdal Editing and Production: Barbara Göbel / Laura Kemmer / Paul Talcott All working papers are available free of charge on our website www.desiguALdades.net. Leal, Claudia and Van Ausdal, Shawn 2013: “Landscapes of Freedom and Inequality: Environmental Histories of the Pacific and Caribbean Coasts of Colombia”, desiguALdades.net Working Paper Series 58, Berlin: desiguALdades.net International Research Network on Interdependent Inequalities in Latin America. The paper was produced by Claudia Leal and Shawn Van Ausdal as a result of discussions at desiguALdades.net in the context of a conference (11/2012) and a workshop (08/2013). desiguALdades.net International Research Network on Interdependent Inequalities in Latin America cannot be held responsible for errors or any consequences arising from the use of information contained in this Working Paper; the views and opinions expressed are solely those of the author or authors and do not necessarily reflect those of desiguALdades.net. -

2 HISTORICAL ROLE of PALMS in HUMAN CULTURE Ancient and Traditional Palm Products
Tropical Palms 13 2 HISTORICAL ROLE OF PALMS IN HUMAN CULTURE Pre-industrial indigenous people of the past as well as of the present have an intimate and direct relationship with the renewable natural resources of their environment. Prior to the Industrial Age, wild and cultivated plants and wild and domesticated animals provided all of the food and most of the material needs of particular groups of people. Looking back to those past times it is apparent that a few plant families played a prominent role as a source of edible and nonedible raw materials. For the entire world, three plant families stand out in terms of their past and present utility to humankind: the grass family (Gramineae), the legume family (Leguminosae) and the palm family (Palmae). If the geographic focus is narrowed to the tropical regions, the importance of the palm family is obvious. The following discussion sets out to provide an overview of the economic importance of palms in earlier times. No single comprehensive study has yet been made of the historical role of palms in human culture, making this effort more difficult. A considerable amount of information on the subject is scattered in the anthropological and sociological literature as part of ethnographic treatments of culture groups throughout the tropics. Moreover, historical uses of products from individual palm species can be found in studies of major economic species such as the coconut or date palms. It should also be noted that in addition to being highly utilitarian, palms have a pivotal role in myth and ritual in certain cultures. -

The Impact of Utilization of Palm Products on the Population Structure of the Vegetable Ivory Palm (Hyphaene Petersiana, Arecace
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/226349494 The impact of utilization of palm products on the population structure of the Vegetable Ivory Palm (Hyphaene petersiana, A.... Article in Economic Botany · October 1995 DOI: 10.1007/BF02863085 CITATIONS READS 26 50 3 authors, including: Sian Sullivan Tracey L Konstant Bath Spa University 6 PUBLICATIONS 61 CITATIONS 106 PUBLICATIONS 1,686 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Disrupted Histories, Recovered Pasts | Histoires Perturbées, Passés Retrouvés View project The Study of Value View project All content following this page was uploaded by Sian Sullivan on 17 February 2016. The user has requested enhancement of the downloaded file. THE IMPACT OF UTILIZATION OF PALM PRODUCTS ON THE POPULA- TION STRUCTURE OF THE VEGETABLE IVORY PALM (HYPHAENE PETERSIANA, ARECACEAE) IN NORTH-CENTRAL NAMIBIA 1 S. SULLIVAN, T. L. KONSTANT, AND A. B. CUNNINGHAM Sullivan, Sian (Department of Anthropology, University College London, Gower Street, London WCIE 6BT, UK), Konstant, Traeey L. (4 Walker Avenue, Discovery, Roodepoort, 1707, South Africa), and Cunninghan-., Anthony B. (PO Box 42, Betty's Bay, 7141, South Africa). THE IMPACTOF UTILIZATIONOF PALMPRODUCTS ON THE POPULATION STRUCTURE OF THE VEGETABLE IVORY PALM (HYPHAENE PETERSIANA) IN NORTH-CENTRAL NAMIBIA. Economic Botany 49(4): 357-370. 1995. Indigenous trees fulfil many subsistence and economic needs in north-central Namibia. Hyphaene petersiana provides a range of products which contribute to most aspects of people's livelihoods. Of particular importance is its income-generating capacity through the use of palm leaves for basket production and the sale of liquor distilled fiom the fruits. -

Resource Assessment of Non-Wood Forest Products
,., NON-WOOD FOREST PRODUCTS 13 - > /1 Resource assessment of non-wood forest · products Experience and biometric principles NON-WOOD FOREST PRODUCTS 13 Resource assessment of non-wood forest products Experience and biometric principles by Jennifer L.G. Wong School of Agricultural and Forest Sciences, University of Wales, Bangor, Gwynedd, UK Kirsti Thornber LTS International, Pentlands Science Park, Bush Loan, Penicuik, Edinburgh, Scotland, UK Nell Baker Tropical Forest Resource Group, South Parks Road, Oxford, UK Department for International D FI D Development FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2001 The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of Food and Agriculture Organization of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication is an output from a research project funded by the United Kingdom Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID. ZF0077 Forestry Research Programme. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying or otherwise, without the prior permission of the copyright owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Information Division, Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. -
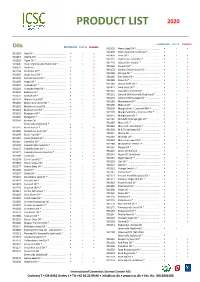
Product List 2020
PRODUCT LIST 2020 CONVENTIONAL ORGANIC STABILIZED Oils CONVENTIONAL ORGANIC STABILIZED 901199 Hemp Seed Oil * .................................. • • • 901499 Hemp Seed Oil Unrefined * ................. • • 901193 Acai Oil * ............................................. • • • 901450 Inchi Oil *............................................. • • • 901367 Alfalfa Oil* ........................................... • • • 901112 Jojoba Oil – Colorless * ........................ • • • 901228 Algae Oil * ........................................... • • • 901110 Jojoba Oil – Golden * ........................... • • • 907440 Aloe Oil (Internally Stabilized)* .......... • • 901162 Kakadu Oil * ........................................ • • • 906221 Amla Oil .............................................. • • 901152 Kalahari Melon Seed Oil * ................... • • • 901148 Andiroba Oil * ..................................... • • • 901168 Karanja Oil * ........................................ • • • 901387 Apple Seed Oil * .................................. • • • 901165 Kiwi Seed Oil * ..................................... • • • 901176 Apricot Kernel Oil * ............................. • • • 901185 Kukui Oil * ........................................... • • • 901195 Argan Oil * ........................................... • • • 901180 Lemon Seed Oil * ................................ • • • 901118 Avocado Oil * ...................................... • • • 901421 Lime Seed Oil * .................................... • • • 901218 Avocado Seed Oil * ............................. -

Perennial Edible Fruits of the Tropics: an and Taxonomists Throughout the World Who Have Left Inventory
United States Department of Agriculture Perennial Edible Fruits Agricultural Research Service of the Tropics Agriculture Handbook No. 642 An Inventory t Abstract Acknowledgments Martin, Franklin W., Carl W. Cannpbell, Ruth M. Puberté. We owe first thanks to the botanists, horticulturists 1987 Perennial Edible Fruits of the Tropics: An and taxonomists throughout the world who have left Inventory. U.S. Department of Agriculture, written records of the fruits they encountered. Agriculture Handbook No. 642, 252 p., illus. Second, we thank Richard A. Hamilton, who read and The edible fruits of the Tropics are nnany in number, criticized the major part of the manuscript. His help varied in form, and irregular in distribution. They can be was invaluable. categorized as major or minor. Only about 300 Tropical fruits can be considered great. These are outstanding We also thank the many individuals who read, criti- in one or more of the following: Size, beauty, flavor, and cized, or contributed to various parts of the book. In nutritional value. In contrast are the more than 3,000 alphabetical order, they are Susan Abraham (Indian fruits that can be considered minor, limited severely by fruits), Herbert Barrett (citrus fruits), Jose Calzada one or more defects, such as very small size, poor taste Benza (fruits of Peru), Clarkson (South African fruits), or appeal, limited adaptability, or limited distribution. William 0. Cooper (citrus fruits), Derek Cormack The major fruits are not all well known. Some excellent (arrangements for review in Africa), Milton de Albu- fruits which rival the commercialized greatest are still querque (Brazilian fruits), Enriquito D. -

10. Non-Wood Forest Products
Non-wood forest products 81 (FAO) Chapter 10 10. Non-wood forest products ABSTRACT Non-wood forest products (NWFP) are a major source of food and income. However, few countries monitor their NWFP systematically, so an accurate global assessment is difficult. This chapter provides a summary of NWFP for which data have been collected and describes the most important NWFP in each region, with estimates of economic value where available. Some of the major problems associated with collecting and analysing data on NWFP are discussed, and suggestions for improving this situation are advanced. INTRODUCTION Non-wood forest products (NWFP)24 play an NWFP have taken place in the last few years, important role in the daily life and well-being of assessment of NWFP and the resources that millions of people worldwide. NWFP include provide them is still a difficult task. This products from forests, from other wooded land difficulty is party attributable to the multitude and and from trees outside the forest. Rural and poor variety of products; the many uses at local, people in particular depend on these products as national and international levels; the multiplicity sources of food, fodder, medicines, gums, resins of disciplines and interests of different ministries and construction materials. Traded products and agencies involved in NWFP assessment and contribute to the fulfilment of daily needs and development; the fact that many NWFP are used provide employment as well as income, or marketed outside traditional economic particularly for rural people and especially structures; and the lack of common terminology women. Internationally traded products, such as and units of measurement. -

Ivory Identification: Introduction ______
Ivory identification: Introduction _____________________________________________________________________________________________________ TABLE OF CONTENTS INTRODUCTION 2 WHAT IS IVORY? 3 THE IVORIES 9 Elephant and Mammoth 9 Walrus 13 Sperm Whale and Killer Whale 15 Narwhal 17 Hippopotamus 19 Wart Hog 21 IVORY SUBSTITUTES 23 NATURAL IVORY SUBSTITUTES 25 Bone 25 Shell 25 Helmeted Hornbill 26 Vegetable Ivory 27 MANUFACTURED IVORY SUBSTITUTES 29 APPENDIX 1 Procedure for the Preliminary Identification 31 of Ivory and Ivory Substitutes APPENDIX 2 List of Supplies and Equipment for Use in the 31 Preliminary Identification of Ivory and Ivory Substitutes GLOSSARY 33 SELECTED REFERENCES 35 COVER: An enhanced photocopy of the Schreger pattern in a cross-section of extant elephant ivory. A concave angle and a convex angle have been marked and the angle measurements are shown. For an explanation of the Schreger pattern and the method for measuring and interpreting Schreger angles, see pages 9 – 10. INTRODUCTION _____________________________________________________________________________________________________ Ivory identification: Introduction Reprinted: 1999 Ivory identification: Introduction 3 _____________________________________________________________________________________________________ The methods, data and background information on ivory identification compiled in this handbook are the result of forensic research conducted by the United States National Fish & Wildlife Forensics Laboratory, located in Ashland, Oregon. The goal of the research