Using Naturally Occurring Terata to Distinguish the Possible from the Impossible in Orchid Floral Evolution Richard M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Native Orchid Society of South Australia
NATIVE ORCHID SOCIETY of SOUTH AUSTRALIA NATIVE ORCHID SOCIETY OF SOUTH AUSTRALIA JOURNAL Volume 6, No. 10, November, 1982 Registered by Australia Post Publication No. SBH 1344. Price 40c PATRON: Mr T.R.N. Lothian PRESIDENT: Mr J.T. Simmons SECRETARY: Mr E.R. Hargreaves 4 Gothic Avenue 1 Halmon Avenue STONYFELL S.A. 5066 EVERARD PARK SA 5035 Telephone 32 5070 Telephone 293 2471 297 3724 VICE-PRESIDENT: Mr G.J. Nieuwenhoven COMMITTFE: Mr R. Shooter Mr P. Barnes TREASURER: Mr R.T. Robjohns Mrs A. Howe Mr R. Markwick EDITOR: Mr G.J. Nieuwenhoven NEXT MEETING WHEN: Tuesday, 23rd November, 1982 at 8.00 p.m. WHERE St. Matthews Hail, Bridge Street, Kensington. SUBJECT: This is our final meeting for 1982 and will take the form of a Social Evening. We will be showing a few slides to start the evening. Each member is requested to bring a plate. Tea, coffee, etc. will be provided. Plant Display and Commentary as usual, and Christmas raffle. NEW MEMBERS Mr. L. Field Mr. R.N. Pederson Mr. D. Unsworth Mrs. P.A. Biddiss Would all members please return any outstanding library books at the next meeting. FIELD TRIP -- CHANGE OF DATE AND VENUE The Field Trip to Peters Creek scheduled for 27th November, 1982, and announced in the last Journal has been cancelled. The extended dry season has not been conducive to flowering of the rarer moisture- loving Microtis spp., which were to be the objective of the trip. 92 FIELD TRIP - CHANGE OF DATE AND VENUE (Continued) Instead, an alternative trip has been arranged for Saturday afternoon, 4th December, 1982, meeting in Mount Compass at 2.00 p.m. -

November December 2010 Vol 67.3 Victoria Natural
NOVEMBER DECEMBER 2010 VOL 67.3 VICTORIA NATURAL HISTORY SOCIETY The Victoria Naturalist Vol. 67.3 (2010) 1 Published six times a year by the SUBMISSIONS VICTORIA NATURAL HISTORY SOCIETY, P.O. Box 5220, Station B, Victoria, BC V8R 6N4 Deadline for next issue: December 1, 2010 Contents © 2010 as credited. Send to: Claudia Copley ISSN 0049—612X Printed in Canada 657 Beaver Lake Road, Victoria BC V8Z 5N9 Editors: Claudia Copley, 250-479-6622, Penelope Edwards Phone: 250-479-6622 Desktop Publishing: Frances Hunter, 250-479-1956 e-mail: [email protected] Distribution: Tom Gillespie, Phyllis Henderson, Morwyn Marshall Printing: Fotoprint, 250-382-8218 Guidelines for Submissions Opinions expressed by contributors to The Victoria Naturalist Members are encouraged to submit articles, field trip reports, natural are not necessarily those of the Society. history notes, and book reviews with photographs or illustrations if possible. Photographs of natural history are appreciated along with VICTORIA NATURAL HISTORY SOCIETY documentation of location, species names and a date. Please label your Honorary Life Members Dr. Bill Austin, Mrs. Lyndis Davis, submission with your name, address, and phone number and provide a Mr. Tony Embleton, Mr. Tom Gillespie, Mrs. Peggy Goodwill, title. We request submission of typed, double-spaced copy in an IBM compatible word processing file on diskette, or by e-mail. Photos and Mr. David Stirling, Mr. Bruce Whittington slides, and diskettes submitted will be returned if a stamped, self- Officers: 2009-2010 addressed envelope is included with the material. Digital images are PRESIDENT: Darren Copley, 250-479-6622, [email protected] welcome, but they need to be high resolution: a minimum of 1200 x VICE-PRESIDENT: James Miskelly, 250-477-0490, [email protected] 1550 pixels, or 300 dpi at the size of photos in the magazine. -

ANPS(A) INDIGENOUS ORCHID STUDY GROUP ISSN 1036-9651 Group Leaders: Don and Pauline Lawie P.O
ANPS(A) INDIGENOUS ORCHID STUDY GROUP ISSN 1036-9651 Group Leaders: Don and Pauline Lawie P.O. Bos 230. BABINDA 4861 Phone: 0740 671 577 News1 etter 7 1 June 2010 I was going to commence this newsletter with apologies to anyone whose photographs were not up to scratch and blame the printer. However, a little more experimentation revealed operator ignorance. The error of having the first page of Newsletter 70, declaring it was Newsletter 69 and dated December 2009, while the other pages are headed correctly, can only be explained by lack of attention to detail by the typist. Yes, that was me and I do apologise.. I also failed to enclose receipts where they were due. More apologies. "Pauline must pay more attention to her home work" C 1950. Eleanor Handreck, Study Groups Liaison Officer for APS, South Australia Region, commented in her report on our newsletter 69: "Why an Oz orchid should have the specific epithet 'sinensis' (Chinese, or from China) is beyond me!" Incase it has occurred to anyoile else to wu~ide~."This widespread species extends from India- to Australia and New Zealand, as far north as China, Japan and Siberia" says BotanicaJAs.Pocket Orchids. The taxonomic convention decrees that the first name given to a plant is THE name, so that if the same plant is found and named elsewhere, when a previous recording is uncovered the new name is changed to the original. As Australia is in the New World it has happened quite a bit to us and it seems a pity that the specific name of some of our orchids no longer honours people we know who made such wonderhl "discoveries". -

A Phylogenomic Analysis of the Floral Transcriptomes of Sexually Deceptive and Rewarding European Orchids, Ophrys and Gymnadenia
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2019 A phylogenomic analysis of the floral transcriptomes of sexually deceptive and rewarding European orchids, Ophrys and Gymnadenia Pineiro Fernandez, Laura ; Byers, Kelsey J R P ; Cai, Jing ; Sedeek, Khalid E M ; Kellenberger, Roman T ; Russo, Alessia ; Qi, Weihong ; Aquino Fournier, Catharine ; Schlüter, Philipp M Abstract: The orchids (Orchidaceae) constitute one of the largest and most diverse families of flowering plants. They have evolved a great variety of adaptations to achieve pollination by a diverse group of pollinators. Many orchids reward their pollinators, typically with nectar, but the family is also well- known for employing deceptive pollination strategies in which there is no reward for the pollinator, in the most extreme case by mimicking sexual signals of pollinators. In the European flora, two examples of these different pollination strategies are the sexually deceptive genus Ophrys and the rewarding genus Gymnadenia, which differ in their level of pollinator specialization; Ophrys is typically pollinated by pseudo-copulation of males of a single insect species, whilst Gymnadenia attracts a broad range of floral visitors. Here, we present and describe the annotated floral transcriptome of Ophrys iricolor, an Andrena- pollinated representative of the genus Ophrys that is widespread throughout the Aegean. Furthermore, we present additional floral transcriptomes of both sexually deceptive and rewarding orchids, specifi- cally the deceptive Ophrys insectifera, Ophrys aymoninii, and an updated floral transcriptome of Ophrys sphegodes, as well as the floral transcriptomes of the rewarding orchids Gymnadenia conopsea, Gym- nadenia densiflora, Gymnadenia odoratissima, and Gymnadenia rhellicani (syn. -

Physiological Features of the Terrestrial Orchids
Anallelle Uniiversiităţiiii diin Craiiova, seriia Agriiculltură – Montanollogiie – Cadastru (Annalls of the Uniiversiity of Craiiova - Agriicullture, Montanollogy, Cadastre Seriies)Voll. XLIX/2019 PHYSIOLOGICAL FEATURES OF THE TERRESTRIAL ORCHIDS CEPHALANTHERA LONGIFOLIA AND PLATANTHERA BIFOLIA THAT GROW IN THE PEDO-CLIMATIC CONDITIONS FROM OLTENIA REGION OF ROMANIA BUSE-DRAGOMIR LUMINITA (1), NICOLAE ION (2), NICULESCU MARIANA (3) (1) University of Craiova, E-mail: [email protected] (2) University of Craiova, E-mail: [email protected] (3) University of Craiova, E-mail: [email protected] *Corresponding author email: [email protected] Key words: terrestrial orchids, transpiration, photosynthesis, light ABSTRACT For studying the physiology of terrestrial orchids, plants from the species Cephalanthera longifolia and Platanthera bifolia have been used. The experiences were carried out directly on the field, in Mehedinţi County, Comanesti Hills. In both species of orchids, the seasonal dynamics of photosynthesis registers a peak during the flowering period that corresponds to a maximum content of assimilating pigments and also a maximum leaf surface. In terrestrial orchids, the seasonal dynamics of leaf transpiration intensity is highest during spring, when the water content in the soil is high and minimal in summer. The graphs showing the diurnal variation of photosynthesis for the two species indicate that Cephalanthera longifolia prefers semi-shaded and sunny habitats, while the plants of Platanthera bifolia that are found in the studied areas prefer a more shaded environment INTRODUCTION Orchids that grow directly at the two, more or less globular or digitally- ground level in large areas of Europe or branched tubules. From the main tuber on the grasslands of the tropical regions comes the flowering stem. -

View .Pdf of This Issue
The Hardy Orchid Society Newsletter No. 21 July 2001 The Hardy Orchid Society Committee is… President: Richard M Bateman Vice-Presidents: Paul Harcourt Davies and Norman Heywood Chairman: Richard Manuel, Wye View Cottage, Leys Hill, Ross-on-Wye, Herefordshire, HR9 5QU Secretary: Sarah Marks, 83 Ladysmith, East Gomeldon, Salisbury, Wilts, SP4 6LE Treasurer: Tony Beresford, Pound Farm, Wearne, Langport, Somerset, TA10 0QJ Membership Secretary: Nick Storer, 17 Orchard Close, Lymm, Cheshire, WA13 9HH Show Secretary: Doreen Webster, 25 Highfields Drive, Loughborough, Leics, LE11 3JS Newsletter Editor: Moira Tarrant, Bumby’s, Fox Rd., Mashbury, Chelmsford, CM1 4TJ Meetings Secretary: Colin Clay, 14 Cromwell Place, Lighthorne Heath, Leamington Spa, CV33 9TG Ordinary Member (publicity): Simon Tarrant, Bumby’s, Fox Rd., Mashbury, Chelmsford, CM1 4TJ Ordinary Member (Newsletter Dist.): Bill Temple, Primrose Cottage, Hanney Rd., Ste- venton, Oxon, OX13 6AP Ordinary Member (Seed & Fungus Bank): Ted Weeks, 74 Over Lane, Almondsbury, Bristol, BS32 4BT Co-opted Member (BOC Rep.): Richard Nicol, 1364 Evesham Rd., Astwood Bank, Red- ditch, Worcs, B96 6BD Contents P.3 From the New President, Richard Bateman P.5 Report of the 9th AGM of the Hardy Orchid Society P.7 Publicity Posters, Simon Tarrant P.8 HELP!, Richard Manuel P.8 HOS Plant Show 2001, Tony Hughes P.10 Does DNA reveal All about the Evolution of Terrestrial Orchids? Part 3, Richard Bateman P.14 Getting Started - the Basics of Hardy Orchid Cultivation, Alan Dash P.20 Chemical Warfare, Richard Manuel P.21 AGS Summer South Show - Silver Award, Carol Dash P.22 Seed and Fungus Bank, Ted Weeks Colour Insert between Pages 12 and 13 Cover illustration: Serapias lingua by Carol Dash HOS Newsletter 21, July 2001 From the New President of the HOS Richard Bateman I am of course delighted to accept the Presidency of the Hardy Orchid Society. -

Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B
Transformations of Lamarckism Vienna Series in Theoretical Biology Gerd B. M ü ller, G ü nter P. Wagner, and Werner Callebaut, editors The Evolution of Cognition , edited by Cecilia Heyes and Ludwig Huber, 2000 Origination of Organismal Form: Beyond the Gene in Development and Evolutionary Biology , edited by Gerd B. M ü ller and Stuart A. Newman, 2003 Environment, Development, and Evolution: Toward a Synthesis , edited by Brian K. Hall, Roy D. Pearson, and Gerd B. M ü ller, 2004 Evolution of Communication Systems: A Comparative Approach , edited by D. Kimbrough Oller and Ulrike Griebel, 2004 Modularity: Understanding the Development and Evolution of Natural Complex Systems , edited by Werner Callebaut and Diego Rasskin-Gutman, 2005 Compositional Evolution: The Impact of Sex, Symbiosis, and Modularity on the Gradualist Framework of Evolution , by Richard A. Watson, 2006 Biological Emergences: Evolution by Natural Experiment , by Robert G. B. Reid, 2007 Modeling Biology: Structure, Behaviors, Evolution , edited by Manfred D. Laubichler and Gerd B. M ü ller, 2007 Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability in Human and Animal Communication , edited by Kimbrough D. Oller and Ulrike Griebel, 2008 Functions in Biological and Artifi cial Worlds: Comparative Philosophical Perspectives , edited by Ulrich Krohs and Peter Kroes, 2009 Cognitive Biology: Evolutionary and Developmental Perspectives on Mind, Brain, and Behavior , edited by Luca Tommasi, Mary A. Peterson, and Lynn Nadel, 2009 Innovation in Cultural Systems: Contributions from Evolutionary Anthropology , edited by Michael J. O ’ Brien and Stephen J. Shennan, 2010 The Major Transitions in Evolution Revisited , edited by Brett Calcott and Kim Sterelny, 2011 Transformations of Lamarckism: From Subtle Fluids to Molecular Biology , edited by Snait B. -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -
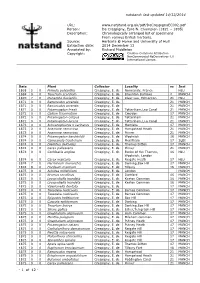
Date Plant Collector Locality Vc Inst 1868 5 0 Primula Polyantha Crespigny, E
natstand: last updated 14/12/2014 URL: www.natstand.org.uk/pdf/DeCrespignyEC002.pdf Person: De Crespigny, Eyre N. Champion (1821 – 1895) Description: Chronologically arranged list of specimens From various British herbaris. Source: Herbaria @ Home and University of Hull Extraction date: 2014 December 13 Annotated by: Richard Middleton Copyright: Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Date Plant Collector Locality vc Inst 1868 5 0 Primula polyantha Crespigny, E. de Normandy, France HLU 1869 0 0 Teucrium scordium Crespigny, E. de Braunton Burrows 4 MANCH 1870 7 0 Oenanthe fluviatilis Crespigny, E. de River Lee, Edmonton 21 HLU 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Ranunculus arvensis Crespigny, E. de 21 MANCH 1871 0 0 Potamogeton friesii Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1872 0 0 Galium tricornutum Crespigny, E. de Croydon 17 MANCH 1872 0 0 Potamogeton crispus Crespigny, E. de Tottenham 21 MANCH 1872 0 0 Potamogeton lucens Crespigny, E. de Tottenham,Lea Canal 21 MANCH 1873 0 0 Schoenoplectus x carinatus Crespigny, E. de Mortlake 17 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Hampstead Heath 21 MANCH 1873 0 0 Anemone nemorosa Crespigny, E. de Pinner 21 MANCH 1874 0 0 Potamogeton berchtoldii Crespigny, E. de Woolwich 16 MANCH 1874 0 0 Campanula trachelium Crespigny, E. de Merstham 17 SLBI 1874 0 0 Dianthus deltoides Crespigny, E. de Thames Ditton 17 MANCH 1874 0 0 Carex pallescens Crespigny, E. de Pinner 21 MANCH 1874 0 0 Cochlearia anglica Crespigny, E. de Banks of the Thames, 16 HLU Woolwich, London 1874 6 0 Carex vesicaria Crespigny, E. -

Review Article Conservation Status of the Family Orchidaceae in Spain Based on European, National, and Regional Catalogues of Protected Species
Hind ile Scientific Volume 2018, Article ID 7958689, 18 pages https://doi.org/10.1155/2018/7958689 Hindawi Review Article Conservation Status of the Family Orchidaceae in Spain Based on European, National, and Regional Catalogues of Protected Species Daniel de la Torre Llorente© Biotechnology-Plant Biology Department, Higher Technical School of Agronomic, Food and Biosystems Engineering, Universidad Politecnica de Madrid, 28140 Madrid, Spain Correspondence should be addressed to Daniel de la Torre Llorente; [email protected] Received 22 June 2017; Accepted 28 December 2017; Published 30 January 2018 Academic Editor: Antonio Amorim Copyright © 2018 Daniel de la Torre Llorente. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Tis report reviews the European, National, and Regional catalogues of protected species, focusing specifcally on the Orchidaceae family to determine which species seem to be well-protected and where they are protected. Moreover, this examination highlights which species appear to be underprotected and therefore need to be included in some catalogues of protection or be catalogued under some category of protection. Te national and regional catalogues that should be implemented are shown, as well as what species should be included within them. Tis report should be a helpful guideline for environmental policies about orchids conservation in Spain, at least at the regional and national level. Around 76% of the Spanish orchid fora are listed with any fgure of protection or included in any red list, either nationally (about 12-17%) or regionally (72%). -

Ecological Developmental Biology and Disease States CHAPTER 5 Teratogenesis: Environmental Assaults on Development 167
Integrating Epigenetics, Medicine, and Evolution Scott F. Gilbert David Epel Swarthmore College Hopkins Marine Station, Stanford University Sinauer Associates, Inc. • Publishers Sunderland, Massachusetts U.S.A. © Sinauer Associates, Inc. This material cannot be copied, reproduced, manufactured or disseminated in any form without express written permission from the publisher. Brief Contents PART 1 Environmental Signals and Normal Development CHAPTER 1 The Environment as a Normal Agent in Producing Phenotypes 3 CHAPTER 2 How Agents in the Environment Effect Molecular Changes in Development 37 CHAPTER 3 Developmental Symbiosis: Co-Development as a Strategy for Life 79 CHAPTER 4 Embryonic Defenses: Survival in a Hostile World 119 PART 2 Ecological Developmental Biology and Disease States CHAPTER 5 Teratogenesis: Environmental Assaults on Development 167 CHAPTER 6 Endocrine Disruptors 197 CHAPTER 7 The Epigenetic Origin of Adult Diseases 245 PART 3 Toward a Developmental Evolutionary Synthesis CHAPTER 8 The Modern Synthesis: Natural Selection of Allelic Variation 289 CHAPTER 9 Evolution through Developmental Regulatory Genes 323 CHAPTER 10 Environment, Development, and Evolution: Toward a New Synthesis 369 CODA Philosophical Concerns Raised by Ecological Developmental Biology 403 APPENDIX A Lysenko, Kammerer, and the Truncated Tradition of Ecological Developmental Biology 421 APPENDIX B The Molecular Mechanisms of Epigenetic Change 433 APPENDIX C Writing Development Out of the Modern Synthesis 441 APPENDIX D Epigenetic Inheritance Systems: -

Actes Du 15E Colloque Sur Les Orchidées De La Société Française D’Orchidophilie
Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie du 30 mai au 1er juin 2009 Montpellier, Le Corum Comité d’organisation : Daniel Prat, Francis Dabonneville, Philippe Feldmann, Michel Nicole, Aline Raynal-Roques, Marc-Andre Selosse, Bertrand Schatz Coordinateurs des Actes Daniel Prat & Bertrand Schatz Affiche du Colloque : Conception : Francis Dabonneville Photographies de Francis Dabonneville & Bertrand Schatz Cahiers de la Société Française d’Orchidophilie, N° 7, Actes du 15e Colloque sur les orchidées de la Société Française d’Orchidophilie. ISSN 0750-0386 © SFO, Paris, 2010 Certificat d’inscription à la commission paritaire N° 55828 ISBN 978-2-905734-17-4 Actes du 15e colloque sur les Orchidées de la Société Française d’Orchidophilie, D. Prat et B. Schatz, Coordinateurs, SFO, Paris, 2010, 236 p. Société Française d’Orchidophilie 17 Quai de la Seine, 75019 Paris Cah. Soc. Fr. Orch., n° 7 (2010) – Actes 15e colloque de la Société Française d’Orchidophilie, Montpellier Préface Ce 15e colloque marque le 40e anniversaire de notre société, celle-ci ayant vu le jour en 1969. Notre dernier colloque se tenait il y a 10 ans à Paris en 1999, 10 ans c’est long, 10 ans c’est très loin. Il fallait que la SFO renoue avec cette traditionnelle organisation de colloques, manifestation qui a contribué à lui accorder la place prépondérante qu’elle occupe au sein des orchidophiles français et de la communauté scientifique. C’est chose faite aujourd’hui. Nombreux sont les thèmes qui font l’objet de communications par des intervenants dont les compétences dans le domaine de l’orchidologie ne sont plus à prouver.