Wood 1 Effect of Food Stress on Obelia Geniculata Zooid Differentiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zoology Diversity of Invertebrates Structure
ZOOLOGY DIVERSITY OF INVERTEBRATES STRUCTURE OF OBELIA --------------------------------------------------------------------------- Introduction: Obelia is worldwide in distribution in the form of plant and is observed in white or light brown in colour in the form of fur in the seas and oceans. It is usually known as Sea-fur. Its name is derived from the Greek word called ‘obelias’,means loaf baked on a spit. • Phylum: Colenterata • Class: Hydrozoa • Order: Hydroida • Sub-order: Leptomedusae • Genus: Obelia • Species: geniculata As it is having three forms like polyp, medusa and blasto style, it is described as trimorphic hydrozoan. Among many species like O. geniculate, O. longissimi, O. bidentata, O. dichotoma. O. geniculate is the most common example studied. Habit and Habitat of Obelia: It is sedentary, marine colonial form present throughout the world on the surfaces of sea weeds, rocks, wooden piles, molluscan shells etc., in the shallow water up to 80-100 meters depth. It is observed in both sexual forms as hydroid colony and asexual form as medusa. Structure of Hydroid Colony: Hydroid colony of obelia is sensitive, transparent consists of horizontal hydrorhiza and vertical hydrocaulus. Hydrorhiza: Hydrorhiza is horizontal thread like root attached to the substratum. It is hollow tube like and gives of vertical branches called hydrocaulus. The tubular part of hydrorhiza are also called stolons. Hydrocaulus: Hydrocaulus are vertical branches arising from hydrorhiza for a length of 2-3 cms. These are also hollow with short lateral branches alternatively in cymose manner. Each alternate branch bears terminal polyp zooids. OBELIA COLONY Each ultimate branch terminates in nutritive zooids called hydranth and axils of the older polyps consists of reproductive zooids called blastostyles or gonangia, thus obelia colony is dimorphic and when gonangia produces saucer shaped buds as a result of asexual reproduction and develops into sexual zooids called medusae, obelia colony becomes trimorphic colony. -

Cnidaria: Hydrozoa) Associated to a Subtropical Sargassum Cymosum (Phaeophyta: Fucales) Bed
ZOOLOGIA 27 (6): 945–955, December, 2010 doi: 10.1590/S1984-46702010000600016 Seasonal variation of epiphytic hydroids (Cnidaria: Hydrozoa) associated to a subtropical Sargassum cymosum (Phaeophyta: Fucales) bed Amanda Ferreira Cunha1 & Giuliano Buzá Jacobucci2 1 Programa de Pós-Graduação em Zoologia, Instituto de Biociências, Universidade de São Paulo. Rua do Matão, Travessa 14, 101, Cidade Universitária, 05508-900 São Paulo, SP, Brazil. E-mail: [email protected] 2 Instituto de Biologia, Universidade Federal de Uberlândia. Rua Ceará, Campus Umuarama, 38402-400 Uberlândia, MG, Brazil. E-mail: [email protected] ABSTRACT. Hydroids are broadly reported in epiphytic associations from different localities showing marked seasonal cycles. Studies have shown that the factors behind these seasonal differences in hydroid richness and abundance may vary significantly according to the area of study. Seasonal differences in epiphytic hydroid cover and richness were evaluated in a Sargassum cymosum C. Agardh bed from Lázaro beach, at Ubatuba, Brazil. Significant seasonal differences were found in total hydroid cover, but not in species richness. Hydroid cover increased from March (early fall) to February (summer). Most of this pattern was caused by two of the most abundant species: Aglaophenia latecarinata Allman, 1877 and Orthopyxis sargassicola (Nutting, 1915). Hydroid richness seems to be related to S. cymosum size but not directly to its biomass. The seasonal differences in hydroid richness and algal cover are shown to be similar to other works in the study region and in the Mediterranean. Seasonal recruitment of hydroid species larvae may be responsible for their seasonal differences in algal cover, although other factors such as grazing activity of gammarid amphipods on S. -

Hydroids and Hydromedusae of Southern Chesapeake Bay
W&M ScholarWorks Reports 1971 Hydroids and hydromedusae of southern Chesapeake Bay Dale Calder Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Marine Biology Commons, Oceanography Commons, Terrestrial and Aquatic Ecology Commons, and the Zoology Commons Recommended Citation Calder, D. (1971) Hydroids and hydromedusae of southern Chesapeake Bay. Special papers in marine science; No. 1.. Virginia Institute of Marine Science, William & Mary. http://doi.org/10.21220/V5MS31 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. LIST OF TABLES Table Page Data on Moerisia lyonsi medusae ginia ...................... 21 rugosa medusae 37 Comparison of hydroids from Virginia, with colonies from Passamaquoddy Bay, New Brunswick.. .................. Hydroids reported from the Virginia Institute of Marine Science (Virginia Fisheries Laboratory) collection up to 1959 ................................................ Zoogeographical comparisons of the hydroid fauna along the eastern United States ............................... List of hydroids from Chesapeake Bay, with their east coast distribution ...me..................................O 8. List of hydromedusae known from ~hesa~eakeBay and their east coast distribution .................................. LIST OF FIGURES Figure Page 1. Southern Chesapeake Bay and adjacent water^.............^^^^^^^^^^^^^^^^^^^^^^^^ 2. Oral view of Maeotias inexpectata ........e~~~~~e~~~~~~a~~~~~~~~~~~o~~~~~~e 3. rature at Gloucester Point, 1966-1967..........a~e.ee~e~~~~~~~aeaeeeeee~e 4. Salinity at Gloucester Point, 1966-1967..........se0me~BIBIeBIBI.e.BIBIBI.BIBIBIs~e~eeemeea~ LIST OF PLATES Plate Hydroids, Moerisia lyonsi to Cordylophora caspia a a e..a a * a 111 ................... -

Phylum Cnidaria-Radiate Animals
Phylum Cnidaria-Radiate Animals • Tissue level of organization • 2 Germ layers • Hydrostatic Skeleton • Gastrovascular Cavity- for digestion • Polymorphism • Polyp (sessile) and Medusa (free-living) stages Class Hydrozoa 1. Lifestyle- Both polyp and medusa stages dominant 2. Reproduction- asexual by budding or sexual 3. 10 tentacles 4. Ex: Obelia, Obelia medusae, Hydra, Hydra reproductive stages Class Scyphozoa- True Jellyfish 1. Lifestyle- Solitary- Medusa-stage dominant 2. Ex: Aurelia, Aurelia lifecycle Class Anthozoa 1. Lifestyle- Polyp stage dominant 2. Gastrovascular cavity divided into mesenteries 3. Ex: Mertidium, Metridium dissection Return to original outline Return to phyla outline Class Hydrozoa Hydra C.S L.S 100X W.M Male Female Return to original outline Return to phyla outline Hydra 40X W.M Return to original outline Return to phyla outline Budding 40X Spermary Ovary 40X Return to original outline Return to phyla outline Obelia Obelia Colony 40X Return to original outline Return to phyla outline Medusae 100x Return to original outline Return to phyla outline Class Scyphozoa Aurelia Lifecycle Planula 100x Schyphistoma 100x Strobila 100x Ephrya 40x Return to original outline Return to phyla outline Aurelia Return to original outline Return to phyla outline Class Anthozoa Metridium LS CS Dissection scope Return to original outline Return to phyla outline Metridium Return to original outline Return to phyla outline Phylum Porifera-The Sponges • Multicellular • Cellular level of organization • No division of labor among cells • No body systems, no organs, no mouth/digestive tract • No germ layers • Pores and canal systems Class Calcarea 1. Spicule type- calcium carbonate 2. Canal system- asconoid, leuconoid and syconoid 3. -

Moodle Interface
Morphology and Physiology of Obelia Discipline Course – I Semester - II Paper : Obelia Lesson: Morphology and Physiology of Obelia Lesson Developer: Sarita Kumar College: Acharya Narendra Dev College University of Delhi Institute of Lifelong Learning, University of Delhi Morphology and Physiology of Obelia Table of Contents • Introduction • Habit and Habitat • Morphology • Hydrorhiza • Hydrocaulus • Living Tissue of Obelia - Coenosarc • Epidermis • Gastrodermis • Protective Covering - Perisarc • Morphology of a Gastrozooid • Morphology of a Gonozooid • Morphology of a Medusa • Locomotion in Obelia • Nutrition in Obelia • Respiration in Obelia • Excretion and Osmoregulation in Obelia • Sense Organ - Statocyst • Reproduction in Obelia • Asexual Reproduction • Sexual Reproduction • Metagenesis • Polymorphism • Summary • Exercise/Practice • Glossary • References/Bibliography/Further Reading Institute of Lifelong Learning, University of Delhi 1 Morphology and Physiology of Obelia Introduction Obelia is a sedentary colonial marine cnidarian which grows upright in a branching tree-like form and has several specialized feeding and reproductive polyps. It is commonly called sea-fur and exists in both asexual, sessile, polypoid stage and sexual, free-swimming medusoid phase. Value Addition: Interesting to Know!! Heading Text: Origin of word ‘Obelia’ Body Text: The word Obelia is probably derived from the Greek word – ‘obeliās’, which means a loaf baked on a spit; obel (ós) - a spit + -ias noun suffix. Source: http://www.answers.com/topic/obelia The common species of Obelia are: a) Obelia geniculata (Knotted thread hydroid) b) Obelia longissima (Sessile hydroid) c) Obelia dichotoma (Sea thread hydroid) d) Obelia bidentata (Double toothed hydroid) Value Addition: Fact File! Heading Text: Different species of Obelia Body Text: Obelia longissima is a long, flexible hydroid colony with a prominent main stem and branches. -
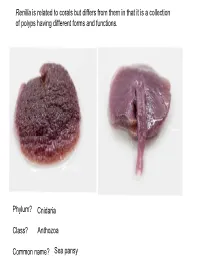
Phylum? Class? Common Name? Cnidaria Anthozoa Sea Pansy
Renilla is related to corals but differs from them in that it is a collection of polyps having different forms and functions. Phylum? Cnidaria Class? Anthozoa Common name? Sea pansy Phylum? Cnidaria Class? Hydrozoa Body form? Polyp Obelia colony Feeding polyp Phylum? Cnidaria Class? Hydrozoa Reproductive polyp Medusa bud – produced asexually Phylum? Cnidaria What is this? This is an Obelia medusa as seen under a compound microscope Class? Hydrozoa How does this reproduce? Sexually by producing eggs or sperm Phylum? Cnidaria Class? Hydrozoa What is the significance of the velum in the taxonomy of this organism? The velum is a characteristic of hydrozoan jellies. Thus Gonionemus is in Class Hydrozoa A B C D A: Exumbrella B: Subumbrella Goniomenus C: Manubrium D: Velum A: Tentacles C: Gonad A B B: Oral arm C Phylum? Cnidaria Identify Cells Class? Hydrozoa Physalia Tentacle Cnidocytes with nematocysts Phylum Cnidaria Class Hydrozoa 2. Identify structure within cell PhysaliaPhylum Tentacle Cnidaria Class Hydrozoa Nematocyst Physalia1. Identify Tentacle cell Cnidocytes with nematocysts Cnidocyte Phylum Cnidaria Class Hydrozoa Physalia Tentacle Cnidocytes with undischarged? Nematocysts Tentacle: Note Cnidocytes Bud (Asexual Reproduction) Phylum? Cnidaria Class Hydrozoa Hydra -Polyp Phylum Cnidaria Class Hydrozoa Obelia What stage of the lifecycle does this represent? Medusa stage (Sexually Reproduces) Phylum Cnidaria Class Hydrozoa Polpys Obelia Colony – A Colony of? Reproductive Polyp Asexual Stage Medusa Bud Feeding Polyp Phylum? Class? Common Name? Cnidaria Hydrozoa Portuguese Man-of-War Common Name? By-the-wind sailor or Velella Common name? Phylum? Class? A B Phylum? Cnidaria Class? Scyphozoa Common Name? Moon Jellly Phylum? Class? Common Name? Cnidaria Anthozoa Sea Anemone Kingdom? Animalia Phylum? Ctenophora Common name? Comb Jelly How does this animal move? Via cilia. -

Polymorphism Lecture No:17 B.Sc Part 1 Zoology(Hons.)
TOPIC: POLYMORPHISM LECTURE NO:17 B.SC PART 1 ZOOLOGY(HONS.)-PAPER I-GROUP A CHAPTER 5 DATE: 15TH MAY 2020 AUTHOR-DR.NIRMAL KUMARI Polymorphism:- Meaning of polymorphism: Occurrence in the same species of more than one type of individuals, which differ in form and function, is known as polymorphism (Gr., Polyps- many or several + Morphe- form). This ensures an efficient division of labor between the several individuals. Two basic forms: In hydrozoa (or coelenterates), which may be single or colonial, there occur two main types of individuals or zooids-polyps and medusa. Fig. Obelia :V.S of Polyp Fig. Medusa in Oral View Patterns of polymorphism: Degree of polymorphism varies greatly in different groups of Hydrozoa. Dimorphic- simplest and commonest pattern of polymorphism is exhibited by many hydrozoan colonies like obelia, tubularia, etc. They have only one type of zooids (individuals). Gastrozooids or hydranths are concerned with feeding, while gonozooids or blastostyles with asexual budding forming sexual medusa or gonophores. Such colonies, bearing only two types of individuals are called dimorphic, and the phenomenon is termed dimorphism. Trimorphic- some forms, like plumularia, are trimorphic. Besides gastrozooids and gonozooids, they also possess a third type of individuals, the dactylozooids.These are functionally non-feeding and defensive polyps bearing batteries of nematocysts. Polymorphic- coelenterates having more than three types of individuals are called polymorphic. Polymorphism is found in the incrusting colony of Hydractinia (Fig.27) and Calycophoran or Siphonophora (Fig.23) with five types of polyps, each performing a specialized function. These are (i) Gastrozooids for feeding, (ii) Spiral dactylozooids for protection, (iii) Long sensory tentaculozooids with sensory cells, (iv) Skeletozooids as spiny projections of chitin, and (v) Gonozooids or reproductive individuals, bearing male or female gonophores (sporosacs) or medusa for sexual reproduction. -

(Marlin) Review of Biodiversity for Marine Spatial Planning Within
The Marine Life Information Network® for Britain and Ireland (MarLIN) Review of Biodiversity for Marine Spatial Planning within the Firth of Clyde Report to: The SSMEI Clyde Pilot from the Marine Life Information Network (MarLIN). Contract no. R70073PUR Olivia Langmead Emma Jackson Dan Lear Jayne Evans Becky Seeley Rob Ellis Nova Mieszkowska Harvey Tyler-Walters FINAL REPORT October 2008 Reference: Langmead, O., Jackson, E., Lear, D., Evans, J., Seeley, B. Ellis, R., Mieszkowska, N. and Tyler-Walters, H. (2008). The Review of Biodiversity for Marine Spatial Planning within the Firth of Clyde. Report to the SSMEI Clyde Pilot from the Marine Life Information Network (MarLIN). Plymouth: Marine Biological Association of the United Kingdom. [Contract number R70073PUR] 1 Firth of Clyde Biodiversity Review 2 Firth of Clyde Biodiversity Review Contents Executive summary................................................................................11 1. Introduction...................................................................................15 1.1 Marine Spatial Planning................................................................15 1.1.1 Ecosystem Approach..............................................................15 1.1.2 Recording the Current Situation ................................................16 1.1.3 National and International obligations and policy drivers..................16 1.2 Scottish Sustainable Marine Environment Initiative...............................17 1.2.1 SSMEI Clyde Pilot ..................................................................17 -

Hydroids (Cnidaria: Hydrozoa) from Japanese Tsunami Marine Debris Washing Ashore in the Northwestern United States
Aquatic Invasions (2014) Volume 9, Issue 4: 425–440 doi: http://dx.doi.org/10.3391/ai.2014.9.4.02 Open Access © 2014 The Author(s). Journal compilation © 2014 REABIC Research Article Hydroids (Cnidaria: Hydrozoa) from Japanese tsunami marine debris washing ashore in the northwestern United States Dale R. Calder1*, Henry H.C. Choong2,3, James T. Carlton4, John W. Chapman5, Jessica A. Miller5 and Jonathan Geller6 1,2Invertebrate Zoology Section, Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, Ontario, Canada, M5S 2C6 3Palaeobiology Section, Department of Natural History, Royal Ontario Museum,100 Queen’s Park, Toronto, Ontario, Canada, M5S 2C6 4Williams College-Mystic Seaport Maritime Studies Program, Mystic, Connecticut 06355, USA 5Department of Fisheries and Wildlife, Hatfield Marine Science Center, Oregon State University, 2030 SE Marine Science Drive, Newport, Oregon 97365, USA 6Moss Landing Marine Laboratories, 8272 Moss Landing Road, Moss Landing, California 95039, USA E-mail: [email protected] (DRC), [email protected] (HHCC), [email protected] (JTC), [email protected] (JWC), [email protected] (JAM), [email protected] (JG) *Corresponding author Received: 12 February 2014 / Accepted: 28 May 2014 / Published online: 4 August 2014 Handling editor: Melissa Frey Abstract Fourteen species of hydroids, including two anthoathecates and 12 leptothecates, are reported from the west coast of North America on debris from the tsunami that struck Japan on 11 March 2011. Six species were found on a dock that stranded at Agate Beach, Newport, Oregon, five from a boat at Gleneden Beach, Oregon, four from a dock in Olympic National Park, Washington, and two from a boat in Grays Harbor, Washington. -
Obelia Longissima Class: Hydrozoa, Hydroidolina
Phylum: Cnidaria Obelia longissima Class: Hydrozoa, Hydroidolina Order: Leptothecata A floating dock hydroid Family: Campanularidae Taxonomy: Obelia longissima was first de- about 0.5 mm in diameter; as they mature, scribed by Pallas in 1766. Synonymous in- they grow to 5 mm in diameter (Cornelius clude Campanularia flabellata, Gonothyraea 1975; Kozloff 1983). longissima, Laomeda flabellata, L. longissi- Color: Medusae are primarily clear. ma, O. flabellata, Sertularia longissima Their tentacle bases, mouths, gonads, and (WoRMS 2015). O. lucifera may also be a stomachs are sometimes yellow to brown, synonym (especially of the medusa form), while their gonads and mouths can be bright but further research is necessary to be sure. green (Puget Sound) (Arai and Brinckmann- There has been much debate over the spe- Voss 1980). cies identities within the genus Obelia Body: (Cornelius 1975; Arai and Brinckmann-Voss Bell: The bell is very thin and flat, with 1980). The taxonomy above was taken from a small stomach, no peduncle, and a rudi- the World Register of Marine Species mentary velum (Fig. 3). It is eversible (Arai (WoRMS 2015). In addition to confusion in and Brinckmann-Voss 1980). the lower taxonomy, the higher taxonomy Radial canals: There are four has undergone revision. The order Hydroida straight radial canals, each containing a glob- was determined to be synonymous with sub- ular gonad (Fig. 3). class Hydroidolina in 2004 (Schuchert Ring canal: The ring canal is 2015). narrow, with eight statocysts (balance struc- tures) (Arai and Brinckmann-Voss 1980) and Description no ocelli (Fig. 3). General Morphology: Obelia longissima Mouth: The mouth has 4 small, has two forms. -

Marine Invasive Species and Biodiversity of South Central Alaska
Marine Invasive Species and Biodiversity of South Central Alaska Anson H. Hines & Gregory M. Ruiz, Editors Smithsonian Environmental Research Center PO Box 28, 647 Contees Wharf Road Edgewater, Maryland 21037-0028 USA Telephone: 443. 482.2208 Fax: 443.482-2295 Email: [email protected], [email protected] Submitted to: Regional Citizens’ Advisory Council of Prince William Sound 3709 Spenard Road Anchorage, AK 99503 USA Telephone: 907.277-7222 Fax: 907.227.4523 154 Fairbanks Drive, PO Box 3089 Valdez, AK 99686 USA Telephone: 907835 Fax: 907.835.5926 U.S. Fish & Wildlife Service 43655 Kalifornski Beach Road, PO Box 167-0 Soldatna, AK 99661 Telephone: 907.262.9863 Fax: 907.262.7145 1 TABLE OF CONTENTS 1. Introduction and Background - Anson Hines & Gregory Ruiz 2. Green Crab (Carcinus maenas) Research – Gregory Ruiz, Anson Hines, Dani Lipski A. Larval Tolerance Experiments B. Green Crab Trapping Network 3. Fouling Community Studies – Gregory Ruiz, Anson Hines, Linda McCann, Kimberly Philips, George Smith 4. Taxonomic Field Surveys A. Motile Crustacea on Fouling Plates– Jeff Cordell B. Hydroids – Leanne Henry C. Pelagic Cnidaria and Ctenophora – Claudia Mills D. Anthozoa – Anson Hines & Nora Foster E. Bryozoans – Judith Winston F. Nemertineans – Jon Norenburg & Svetlana Maslakova G. Brachyura – Anson Hines H. Molluscs – Nora Foster I. Urochordates and Hemichordates – Sarah Cohen J. Echinoderms –Anson Hines & Nora Foster K. Wetland Plants - Dennis Whigham 2 Executive Summary This report summarizes research on nonindigenous species (NIS) in marine ecosystems of Alaska during the year 2000 by the Smithsonian Environmental Research Center. The project is an extension of three years of research on NIS in Prince William Sound, which is presented in a major report (Hines and Ruiz, 2000) that is on line at the website of the Regional Citizens’ Advisory Council: www.pwsrcac.org. -

PHYLUM 'Sea Walnuts'/'Comb Jellies' CTENOPHORA
PHYLUM ‘Sea walnuts’/‘Comb jellies’ CTENOPHORA TISSUE level of body org. RADIAL Symmetry Bodies often transparent &/or luminescent Locomotion = most are free-swimming 8 rows of ciliated combs = ctenes for locomotion Facts: Colloblasts = adhesive cells No nematocysts of their own, although some species gather them from their food! Complete digestive system – mouth to anus PHYLUM CNIDARIA • TISSUE level of body organization • Middle layer = MESOGLEA = Acellular matrix (Just jelly!) • Diagnostic cell type = CNIDOCYTE It contains the nematocyst organelle How many tissue layers? C B ? A A = ? ? B = ? ? C = ? ?? = Lumen of what ? Function? Insert: A Cnidocyte – cell containing a Nematocyst - organelle not yet triggered. C A B Cnidarians are GVC DIPLOBLASTIC Gastro-vascular Cavity (2 tissue layers) C = Epidermis & = coelenteron A = Gastrodermis with B = Mesoglea in between the two Phylum Cnidaria Cnidocil = trigger Undischarged barbs Thread with stylet Cnidae = nematocyst Cnidocyte with nucleus Specialized cells called cnidocytes contain capsule-lie organelles called cnidae…some of which are nematocysts. (Other cnidae have other names and functions). Nematocysts bear thread-like stinging projectiles used for anchorage, defense and prey capture. Cnidarian Life Cycles CLASS • Hydrozoa Polyp dominant ……Medusa does exist (Hydra is cute but odd!) Remember the Hydrocorals! • Scyphozoa Medusa dominant ……Polyp may exist Cubozoa Medusa dominant Polyp inconspicuous or unknown Staurozoa No medusa per se but a medusa-like top exists on a polyp base • Anthozoa Polyp only Can you differentiate between a gonad and a bud? Which is for sexual reproduction? ? ? Cnidocyte-bearing tentacles, mouth & hypostome, GVC (coelenteron) & bud [fig 2.2] A ? B ? Theca made of ? Obelia colony slide with close-up of the some of the polyps or zooids.