Benthic Hydrozoans As Potential Indicators of Water Masses and Anthropogenic Impact in the Sea of Marmara
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mediterranean Marine Science
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by National Documentation Centre - EKT journals Mediterranean Marine Science Vol. 19, 2018 Benthic Hydrozoans as Potential Indicators of Water Masses and Anthropogenic Impact in the Sea of Marmara TOPÇU NUR Istanbul University, Faculty of Aquatic Sciences, Istanbul, Turkey MARTELL LUIS YILMAZ IZZET ISINIBILIR MELEK https://doi.org/10.12681/mms.15117 Copyright © 2018 Mediterranean Marine Science To cite this article: TOPÇU, N., MARTELL, L., YILMAZ, I., & ISINIBILIR, M. (2018). Benthic Hydrozoans as Potential Indicators of Water Masses and Anthropogenic Impact in the Sea of Marmara. Mediterranean Marine Science, 19(2), 273-283. doi:https://doi.org/10.12681/mms.15117 http://epublishing.ekt.gr | e-Publisher: EKT | Downloaded at 07/06/2020 15:19:04 | Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available online at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.15117 Benthic Hydrozoans as Potential Indicators of Water Masses and Anthropogenic Impact in the Sea of Marmara NUR EDA TOPÇU1, LUIS FELIPE MARTELL1, 2, IZZET NOYAN YILMAZ3 and MELEK ISINIBILIR1 1Department of Marine Biology, Faculty of Aquatic Sciences, Istanbul University, Turkey 2University Museum of Bergen, Department of Natural History, University of Bergen, Norway 3Institute of Marine Sciences and Management, Istanbul University, Turkey Corresponding author: [email protected] Handling Editor: Carlo Bianchi Received: 27 November 2017; Accepted: 23 March 2018; Published on line: 18 June 2018 Abstract Changes in the abundance and distribution of marine benthic hydrozoan species are indicative of variations in environmental conditions in the marine realm. -

Hydrozoa of the Eurasian Arctic Seas 397 S
THE ARCTIC SEAS CI imatology, Oceanography, Geology, and Biology Edited by Yvonne Herman IOm51 VAN NOSTRAND REINHOLD COMPANY ~ -----New York This work relates to Department of the Navy Grant NOOOI4-85- G-0252 issued by the Office of Naval Research. The United States Government has a royalty-free license throughout the world in all copyrightable material contained herein. Copyright © 1989 by Van Nostrand Reinhold Softcover reprint of the hardcover 1st edition 1989 Library of Congress Catalog Card Number 88-33800 ISBN-13 :978-1-4612-8022-4 e-ISBN-13: 978-1-4613-0677-1 DOI: 10.1007/978-1-4613-0677-1 All rights reserved. No part of this work covered by the copyright hereon may be reproduced or used in any form or by any means-graphic, electronic, or mechanical, including photocopying, recording, taping, or information storage and retrieval systems-without written permission of the publisher. Designed by Beehive Production Services Van Nostrand Reinhold 115 Fifth Avenue New York, New York 10003 Van Nostrand Reinhold (International) Limited 11 New Fetter Lane London EC4P 4EE, England Van Nostrand Reinhold 480 La Trobe Street Melbourne, Victoria 3000, Australia Nelson Canada 1120 Birchmount Road Scarborough, Ontario MIK 5G4, Canada 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 Library of Congress Cataloging in Publication Data The Arctic Seas. Includes index. 1. Oceanography-Arctic Ocean. 2. Geology-ArctiC Ocean. 1. Herman, Yvonne. GC401.A76 1989 551.46'8 88-33800 ISBN-13: 978-1-4612-8022-4 For Anyu Contents Preface / vii Contributors / ix 1. -

Zoology Diversity of Invertebrates Structure
ZOOLOGY DIVERSITY OF INVERTEBRATES STRUCTURE OF OBELIA --------------------------------------------------------------------------- Introduction: Obelia is worldwide in distribution in the form of plant and is observed in white or light brown in colour in the form of fur in the seas and oceans. It is usually known as Sea-fur. Its name is derived from the Greek word called ‘obelias’,means loaf baked on a spit. • Phylum: Colenterata • Class: Hydrozoa • Order: Hydroida • Sub-order: Leptomedusae • Genus: Obelia • Species: geniculata As it is having three forms like polyp, medusa and blasto style, it is described as trimorphic hydrozoan. Among many species like O. geniculate, O. longissimi, O. bidentata, O. dichotoma. O. geniculate is the most common example studied. Habit and Habitat of Obelia: It is sedentary, marine colonial form present throughout the world on the surfaces of sea weeds, rocks, wooden piles, molluscan shells etc., in the shallow water up to 80-100 meters depth. It is observed in both sexual forms as hydroid colony and asexual form as medusa. Structure of Hydroid Colony: Hydroid colony of obelia is sensitive, transparent consists of horizontal hydrorhiza and vertical hydrocaulus. Hydrorhiza: Hydrorhiza is horizontal thread like root attached to the substratum. It is hollow tube like and gives of vertical branches called hydrocaulus. The tubular part of hydrorhiza are also called stolons. Hydrocaulus: Hydrocaulus are vertical branches arising from hydrorhiza for a length of 2-3 cms. These are also hollow with short lateral branches alternatively in cymose manner. Each alternate branch bears terminal polyp zooids. OBELIA COLONY Each ultimate branch terminates in nutritive zooids called hydranth and axils of the older polyps consists of reproductive zooids called blastostyles or gonangia, thus obelia colony is dimorphic and when gonangia produces saucer shaped buds as a result of asexual reproduction and develops into sexual zooids called medusae, obelia colony becomes trimorphic colony. -

Clearance Rates of Jellyfish and Their Potential Predation Impact on Zooplankton and Fish Larvae in a Neritic Ecosystem (Limfjorden, Denmark)
MARINE ECOLOGY PROGRESS SERIES Vol. 304: 117–131, 2005 Published December 8 Mar Ecol Prog Ser Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark) Lars Johan Hansson1,*, Ole Moeslund2, Thomas Kiørboe1, Hans Ulrik Riisgård2 1Danish Institute for Fisheries Research, Kavalergården 6, 2920 Charlottenlund, Denmark 2Marine Biological Research Centre (University of Southern Denmark) Hindsholmvej 11, 5300 Kerteminde, Denmark ABSTRACT: Clearance rates of the hydromedusae Sarsia tubulosa, Rathkea octopunctata and Bougainvillea superciliaris and the scyphomedusa Aurelia aurita were measured in the laboratory. Gut contents analyses of A. aurita were also collected in situ and subsequently used for estimation of clearance rate. The clearance rate of A. aurita varied widely with prey organisms. Large crustacean prey with low escape capabilities (Artemia salina nauplii and cirripede larvae) were cleared at high rates, whereas copepodites were cleared at lower rates, and clearance rates of small bivalve larvae and copepod nauplii were comparatively low. These data were used to assess the impact of jellyfish preda- tion upon zooplankton and fish larvae in Limfjorden, Denmark. Repeated sampling of zooplankton, fish larvae and medusae was undertaken during the first half of 2003. Nine taxa of hydromedusae and 4 taxa of scyphomedusae were identified. Abundance estimates were combined with estimated clear- ance rates of individual medusae to calculate potential jellyfish-induced mortality on prey in Limfjor- den. Copepoda was used as a model prey group to estimate the collective predation impact by all medusae. Medusa species with unknown clearance potential were given assumed clearance rate val- ues, but the collective predation potential by these species was evaluated to be small. -

Origin of Chordates Part-I
ORIGIN OF CHORDATES Evolution of chordate was one of the most important event in the history of chordate as it was the beginning of evolution of more advanced chordates like bird and mammals. It is the story of origin from a primitive invertebrate like creatures to early chordates. Though first fossil of the first vertebrate the ostrachoderm was discovered from the Ordovician period but it might have originated in late Cambrian period. As early chordates were soft bodied, their fossil records are not preserved. Hence, to trace their ancestry, we have to find out the similarity among different deuterostomes to trace the origin of chordates. Some structural features shared by them such as bilateral symmetry, antero-posterior body axis, triploblastic coelomate condition, etc., may he because of their common ancestry. TIME OF ORIGIN: Late Cambrian period PLACE OF ORIGIN: Fresh water THEORIES OF INVERTEBRATE ANCESTRY OF CHORDATES Several theories have been put forwarded to explain the origin of chordates either directly from some invertebrate group or through the intervention of some protochordate. Almost every invertebrate phylum—Coelenterata, Nemertean, Phoronida, Annelids, Arthropods and Echinodermatashas been suggested. But these theories are far from being satisfactory and convincing and have only a historical value. Only the echinoderm theory has received some acceptance. DIVISION OF BILATERIA The Bilateria is divided into two major divisions (1) Protostomia and (2) Deuterostornia. This division is based on the differences in embryonic and larval developments. Protostomia includes from Annelida to Arthropoda while deuterostomia includes Echinodermata, Pogonophora and Chordate. DEUTEROSTOME LINE OF CHORDATE EVOLUTION Following common features of all Deuterostomes suggests strong evidence of a closer evolutionary relationship between the three principal deuterostome phyla – Echinodermata, Hemichordata and Chordata. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

SCAMIT Newsletter Vol. 15 No. 11 1997 March
March, 1997 SCAMIT Newsletter vol. 15, No. 11 NEXT MEETING: Workshop - The Taxonomy of Benthic Cnidaria GUEST SPEAKER: Moderator - John Ljubenkov DATE: 10-11 April 1997 TIME: 9am - 4pm each day LOCATION: Dancing Coyote Ranch, 20355 Hwy 76, Pauma Valley, California APRIL 10-11 WORKSHOP Our April meeting has been replaced with a two day workshop titled Taxonomy of Benthic Cnidaria emphasizing the fauna of So. California and adjacent regions. Sessions on Hydrozoa and Anthozoa are planned, with particular attention to Polyorchis, corymorphine hydroids and their medusae, Plumularia, burrowing anemones, gorgonians, sea pens and other octocorals. Bring problem specimens, of which there should be no lack. Please contact John @ 619)742-2238 for directions, information, to indicate attendance, or for help arranging accommodations for Polyorchis (from Hyman, 1940. The overnighting. Invertebrates, Volume 1 - Protozoa-Ctenophora) FUNDS FOR THIS PUBLICATION PROVIDED, IN PART, BY THE ARCO FOUNDATION, CHEVRON USA, AND TEXACO INC. SCAMIT Newsletter is not deemed to be a valid publication for formal taxonomic purposes. March, 1997 SCAMIT Newsletter Vol. 15, No. 11 NEW LITERATURE to be a recurrent feature involving several gadoid fishes, with records from 1959, 1970, 1983, and A variety of new papers were distributed at the 19th century European waters. Happily the meeting for member examination. Two dealt with shrimp population recovered within one year in echinoderms, which form the backbone of the most recent episode. Taxonomic Atlas Volume 14, our discussion topic for the meeting. Both concerned holothuroids, Krueger & Cavanaugh (1997) discuss a closer with Rodgers & Bingham (1996) addressing the relationship between two disparate populations; subtidal zonation of the eastern Pacific Cucumaria that of species in the clam genus Solemya and lubrica, and Foster & Hodgson (1996) examining their bacterial symbionts. -

The First Record of Bougainvillia Principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea
Invertebrate Zoology, 2018, 15(4): 333–339 © INVERTEBRATE ZOOLOGY, 2018 The first record of Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea A.A. Prudkovsky1, T.V. Neretina2,3 1 Dept. Invertebrate Zoology, Faculty of Biology, Lomonosov Moscow State University, Leninskie Gory 1–12, 119991 Moscow, Russia. E-mail: [email protected] 2 Pertsov White Sea Biological Station, Biological Faculty, Moscow State University M.V. Lomonos- ov, Leninskie Gory 1-12, 119991 Moscow, Russia. 3 Pirogov Russian National Research Medical University, Ostrovitianov 1, 117997 Moscow, Russia. ABSTRACT: Hydroids are common components of fouling communities in the sea, but they are often inconspicuous and easily overlooked. In such cases, the appearance of their medusae in plankton is an obvious indicator of the species’ presence in a locality. In this study, we present the first record of medusae Bougainvillia principis from the White Sea. We hypothesize that hydroids of the species B. principis inhabit the White Sea, as well, but they do not usually produce medusae and consequently the species does not exhibit sexual reproduction in the White Sea. How to cite this article: Prudkovsky A.A., Neretina T.V. 2018. The first record of Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) from the White Sea // Invert. Zool. Vol.15. No.4. P. 333–339. doi: 10.15298/invertzool.15.4.02 KEY WORDS: Bougainvillia principis, medusa, first report, White Sea. Первая находка медузы Bougainvillia principis (Steenstrup, 1850) (Hydrozoa: Anthoathecata) в Белом море А.A. Прудковский1, Т.В. Неретина2,3 1 Кафедра зоологии беспозвоночных, Биологический факультет МГУ имени М.В. -

Cnidaria: Hydrozoa) Associated to a Subtropical Sargassum Cymosum (Phaeophyta: Fucales) Bed
ZOOLOGIA 27 (6): 945–955, December, 2010 doi: 10.1590/S1984-46702010000600016 Seasonal variation of epiphytic hydroids (Cnidaria: Hydrozoa) associated to a subtropical Sargassum cymosum (Phaeophyta: Fucales) bed Amanda Ferreira Cunha1 & Giuliano Buzá Jacobucci2 1 Programa de Pós-Graduação em Zoologia, Instituto de Biociências, Universidade de São Paulo. Rua do Matão, Travessa 14, 101, Cidade Universitária, 05508-900 São Paulo, SP, Brazil. E-mail: [email protected] 2 Instituto de Biologia, Universidade Federal de Uberlândia. Rua Ceará, Campus Umuarama, 38402-400 Uberlândia, MG, Brazil. E-mail: [email protected] ABSTRACT. Hydroids are broadly reported in epiphytic associations from different localities showing marked seasonal cycles. Studies have shown that the factors behind these seasonal differences in hydroid richness and abundance may vary significantly according to the area of study. Seasonal differences in epiphytic hydroid cover and richness were evaluated in a Sargassum cymosum C. Agardh bed from Lázaro beach, at Ubatuba, Brazil. Significant seasonal differences were found in total hydroid cover, but not in species richness. Hydroid cover increased from March (early fall) to February (summer). Most of this pattern was caused by two of the most abundant species: Aglaophenia latecarinata Allman, 1877 and Orthopyxis sargassicola (Nutting, 1915). Hydroid richness seems to be related to S. cymosum size but not directly to its biomass. The seasonal differences in hydroid richness and algal cover are shown to be similar to other works in the study region and in the Mediterranean. Seasonal recruitment of hydroid species larvae may be responsible for their seasonal differences in algal cover, although other factors such as grazing activity of gammarid amphipods on S. -

Ferdinando Boero Disteba (Dipartimento Di Scienze E
CV of Ferdinando Boero Contacts: Ferdinando Boero DiSTeBA (Dipartimento di Scienze e Tecnologie Biologiche e Ambientali) Università del Salento 73100 Lecce Italy Voice: -39 0832 298619 Handphone: 3332144956 Fax: -39 0832 298702 or 298626 email: [email protected] 1951, February 13th, born in Genova, Italy. Current position: Full Professor of Zoology and Anthropology at the Department of Biological and Environmental Sciences and Technologies of the University of Salento. Associate at the Istituto di Scienze Marine of the National Research Council Appointments 1988-today, responsible of the Museum and Station of Marine Biology of Porto Cesareo. 1990- today, Member, or past member, of the editorial board of the journals: Advances in Limonology and Oceanography Aquatic Biology Aquatic Invasions Cahiers de Biologie Marine Ecology Letters Italian Journal of Zoology (editor in chief since 2008) Journal of Evolutionary Biology F1000 Research Oebalia Thalassia Salentina 1995-today, member of the Council of the "Consorzio Nazionale Interuniversitario per le Scienze del Mare" (National Interuniversity Consortium for Marine Sciences) (CoNISMa). 1995-today, director of the CoNISMA Research Unit of Lecce. 1996-1998, Member of the Academic Senate of the University of Lecce. 1998-2004, Chair of the Littoral Environments Committee of the International Commission for the Scientific Exploration of the Mediterranean Sea. 2007-2013, Chair of the Biological Resources Committee of the International Commission for the Scientific Exploration of the Mediterranean Sea. 1998-2004, Secretary General of the Società Italiana di Ecologia. 2004-2008, Member of the council of the Società Italiana di Ecologia, 2004-today, Member of the council of the Unione Zoologica Italiana. 2000-2008, responsible, with L. -
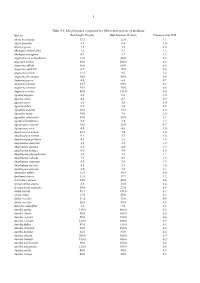
PDF (Table·S1. Morphospace Computed for 660 Extant Species Of
1 Table·S1. Morphospace computed for 660 extant species of medusae Species Bell height H (mm) Bell diameter D (mm) Fineness ratio H/D Abyla bicarinata 13.3 12.4 1.1 Abyla haeckeli 5.8 6.0 1.0 Abyla trigona 7.5 5.1 1.5 Abylopsis eschscholtzi 7.6 5.7 1.3 Abylopsis tetragona 4.9 3.7 1.3 Aeginodiscus actinodiscus 13.0 40.0 0.3 Aeginura beebei 18.0 100.0 0.2 Aequorea albida 30.0 60.0 0.5 Aequorea australis 6.7 18.0 0.4 Aequorea conica 11.0 9.0 1.2 Aequorea floridiana 14.8 40.0 0.4 Aequorea parva 4.0 6.0 0.7 Aequorea tennuis 25.7 90.0 0.3 Aequorea victoria 35.0 70.0 0.5 Aequorea vitrina 50.0 135.0 0.4 Agalma elegans 8.8 8.6 1.0 Agalma okeni 4.4 4.7 0.9 Agastra mira 1.0 1.0 1.0 Agastra rubra 0.8 1.0 0.8 Aglantha digitale 30.0 15.0 2.0 Aglantha ignea 14.0 7.0 2.0 Aglantha intermedia 15.0 14.0 1.1 Aglaura hemistoma 5.0 3.5 1.4 Aglauropsis conanti 15.0 22.0 0.7 Aglauropsis jarli 4.0 4.0 1.0 Amphicaryon acaule 10.3 7.4 1.4 Amphicaryon ernesti 9.2 5.7 1.6 Amphicaryon peltifera 4.3 2.6 1.6 Amphinema australis 3.0 2.5 1.2 Amphinema dinema 6.0 4.0 1.5 Amphinema krampi 6.0 4.0 1.5 Amphinema physophorum 2.0 1.8 1.1 Amphinema rubrum 7.0 4.5 1.6 Amphinema rugusum 5.0 3.0 1.7 Amphinema turrida 5.5 5.3 1.0 Amphogona apicata 8.0 7.0 1.1 Annatiara affinis 12.0 14.5 0.8 Apolemia uvaria 21.8 17.7 1.2 Archirhiza aurosa 14.0 40.0 0.4 Arctapodema ampla 5.6 15.0 0.4 Arctapodema australis 14.0 23.0 0.6 Atolla bairdii 46.7 144.0 0.3 Atolla chuni 15.0 50.0 0.3 Atolla wyvillei 31.5 73.0 0.4 Atolla wyvillei 26.0 55.0 0.5 Atorella vanhoffeni 3.0 7.0 0.4 Aurelia aurita -

Geology of Saipan Mariana Islands Part 4
Geology of Saipan Mariana Islands Part 4. Submarine Topography and Shoal- Water Ecology GEOLOGICAL SURVEY PROFESSIONAL PAPER 280-K Geology of Saipan Mariana Islands Part 4. Submarine Topography and Shoal- Water Ecology By PRESTON E. CLOUD, Jr. GEOLOGICAL SURVEY PROFESSIONAL PAPER 280-K Description and interpretation of the submarine topography and of the sediments^ biotas^ and morphology of the reef complex adjacent to a geologically diverse tropical island UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1959 UNITED STATES DEPARTMENT OF THE INTERIOR FRED A. S EATON, Secretary GEOLOGICAL SURVEY Thomas B. Nolan, Director For sale by the Superintendent of Documents, U. S. Government Printing Office Washington 25, D. C. GEOLOGICAL SURVEY PROFESSIONAL PAPER 280 Geology of Saipan, Mariana Islands Part 1. General Geology A. General Geology By PRESTON E. CLOUD, Jr., ROBERT GEORGE SCHMIDT, and HAROLD W. BURKE Part 2. Petrology and Soils B. Petrology of the Volcanic Rocks By ROBERT GEORGE SCHMIDT C. Petrography of the Limestones By J. HARLAN JOHNSON D. Soils By RALPH J. McCRACKEN Part 3. Paleontology E. Calcareous Algae By J. HARLAN JOHNSON F. Difcoaster and Some Related Microfossils By M. N. BRAMLETTE G. Eocene Radiolaria By WILLIAM RIEDEL H. Smaller Foraminifera By RUTH TODD I. Larger Foraminifera By W. STORRS COLE J. Echinoids By C. WYTHE COOKE Part 4. Submarine Topography and Shoal-Water Ecology K. Submarine Topography and Shoal-Water Ecology By PRESTON E. CLOUD, Jr. CONTENTS Page Page Abstract_________________________________________ 361 Shoal-water and shoreline ecology and sediments—Con. Introduction. ______________________________________ 362 Habitat descriptions—Con. Purpose and scope of the work_____________________ 362 Organic reefs and reef benches______________ 383 Field methods and acknowledgments-_______________ 362 Minor reef structures______________________ 384 Systematic identifications and other research aid____ 363 Biotope X.