Status of Red Panda in Sikkim: a Case Study in East Sikkim
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phytophthora Ramorum Sudden Oak Death Pathogen
NAME OF SPECIES: Phytophthora ramorum Sudden Oak Death pathogen Synonyms: Common Name: Sudden Oak Death pathogen A. CURRENT STATUS AND DISTRIBUTION I. In Wisconsin? 1. YES NO X 2. Abundance: 3. Geographic Range: 4. Habitat Invaded: 5. Historical Status and Rate of Spread in Wisconsin: 6. Proportion of potential range occupied: II. Invasive in Similar Climate YES NO X Zones United States: In 14 coastal California Counties and in Curry County, Oregon. In nursery in Washington. Canada: Nursery in British Columbia. Europe: Germany, the Netherlands, the United Kingdom, Poland, Spain, France, Belgium, and Sweden. III. Invasive in Similar Habitat YES X NO Types IV. Habitat Affected 1. Habitat affected: this disease thrives in cool, wet climates including areas in coastal California within the fog belt or in low- lying forested areas along stream beds and other bodies of water. Oaks associated with understory species that are susceptible to foliar infections are at higher risk of becoming infected. 2. Host plants: Forty-five hosts are regulated for this disease. These hosts have been found naturally infected by P. ramorum and have had Koch’s postulates completed, reviewed and accepted. Approximately fifty-nine species are associated with Phytophthora ramorum. These species are found naturally infected; P. ramorum has been cultured or detected with PCR but Koch’s postulates have not been completed or documented and reviewed. Northern red oak (Quercus rubra) is considered an associated host. See end of document for complete list of plant hosts. National Risk Model and Map shows susceptible forest types in the mid-Atlantic region of the United States. -

2. ACER Linnaeus, Sp. Pl. 2: 1054. 1753. 枫属 Feng Shu Trees Or Shrubs
Fl. China 11: 516–553. 2008. 2. ACER Linnaeus, Sp. Pl. 2: 1054. 1753. 枫属 feng shu Trees or shrubs. Leaves mostly simple and palmately lobed or at least palmately veined, in a few species pinnately veined and entire or toothed, or pinnately or palmately 3–5-foliolate. Inflorescence corymbiform or umbelliform, sometimes racemose or large paniculate. Sepals (4 or)5, rarely 6. Petals (4 or)5, rarely 6, seldom absent. Stamens (4 or 5 or)8(or 10 or 12); filaments distinct. Carpels 2; ovules (1 or)2 per locule. Fruit a winged schizocarp, commonly a double samara, usually 1-seeded; embryo oily or starchy, radicle elongate, cotyledons 2, green, flat or plicate; endosperm absent. 2n = 26. About 129 species: widespread in both temperate and tropical regions of N Africa, Asia, Europe, and Central and North America; 99 species (61 endemic, three introduced) in China. Acer lanceolatum Molliard (Bull. Soc. Bot. France 50: 134. 1903), described from Guangxi, is an uncertain species and is therefore not accepted here. The type specimen, in Berlin (B), has been destroyed. Up to now, no additional specimens have been found that could help clarify the application of this name. Worldwide, Japanese maples are famous for their autumn color, and there are over 400 cultivars. Also, many Chinese maple trees have beautiful autumn colors and have been cultivated widely in Chinese gardens, such as Acer buergerianum, A. davidii, A. duplicatoserratum, A. griseum, A. pictum, A. tataricum subsp. ginnala, A. triflorum, A. truncatum, and A. wilsonii. In winter, the snake-bark maples (A. davidii and its relatives) and paper-bark maple (A. -

Number 3, Spring 1998 Director’S Letter
Planning and planting for a better world Friends of the JC Raulston Arboretum Newsletter Number 3, Spring 1998 Director’s Letter Spring greetings from the JC Raulston Arboretum! This garden- ing season is in full swing, and the Arboretum is the place to be. Emergence is the word! Flowers and foliage are emerging every- where. We had a magnificent late winter and early spring. The Cornus mas ‘Spring Glow’ located in the paradise garden was exquisite this year. The bright yellow flowers are bright and persistent, and the Students from a Wake Tech Community College Photography Class find exfoliating bark and attractive habit plenty to photograph on a February day in the Arboretum. make it a winner. It’s no wonder that JC was so excited about this done soon. Make sure you check of themselves than is expected to seedling selection from the field out many of the special gardens in keep things moving forward. I, for nursery. We are looking to propa- the Arboretum. Our volunteer one, am thankful for each and every gate numerous plants this spring in curators are busy planting and one of them. hopes of getting it into the trade. preparing those gardens for The magnolias were looking another season. Many thanks to all Lastly, when you visit the garden I fantastic until we had three days in our volunteers who work so very would challenge you to find the a row of temperatures in the low hard in the garden. It shows! Euscaphis japonicus. We had a twenties. There was plenty of Another reminder — from April to beautiful seven-foot specimen tree damage to open flowers, but the October, on Sunday’s at 2:00 p.m. -

Abies Concolor (White Fir)
Compiled here is distribution, characteristics and other information on host species featured as ‘Host of the Month’ in past issues of the COMTF Monthly Report. Abies concolor (white fir) This is an evergreen tree native to the mountains of southern Oregon, California, the southern Rocky Mountains, and Baja California. Large and symmetrical, white fir grows 80 – 120ft tall and 15 – 20ft wide in its native range and in the Pacific Northwest. White fir is one of the top timber species found in the Sierra Nevada Mountains of CA and is a popular Christmas tree, as well as one of the most commonly grown native firs in Western gardens. Young trees are conical in shape, but develop a dome-like crown with age. The flattened needles of white fir are silvery blue-green, blunt at the tip , and grow 2 – 3in long. Often curving upwards, the needles extend at right angles from the twig, and twigs produce a citrus smell when needles are broken. White fir is monoecious, producing yellow- to red-toned, catkin-like male flowers and inconspicuous yellow-brown female flowers. The oblong cones grow 3 – 5 in upright, are yellow-green to purple in color, and are deciduous at maturity, dispersing seed in the fall. New twigs are dark- orange, but become gray-green, then gray with maturity. The bark of saplings is thin, smooth, and gray, turning thick, ash-gray with age, and developing deep irregular furrows. P. ramorum- infected Abies concolor (white fir) was first reported in the October 2005 COMTF newsletter as having been found at a Christmas tree farm in the quarantined county of Santa Clara. -

The Red List of Revised and Extended
AcerThe Red List of revised and extended Dan Crowley, Megan Barstow, Malin Rivers & Yvette Harvey-Brown BOTANIC GARDENS CONSERVATION INTERNATIONAL (BGCI) is the world’s largest plant conservation network, comprising more than 500 botanic gardens in over 100 countries, and provides the secretariat to the IUCN/SSC Global Tree Specialist Group. BGCI was established in 1987 and is a registered charity with offices in the UK, US, China and Kenya. Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK. © 2020 Botanic Gardens Conservation International THE IUCN/SSC GLOBAL TREE SPECIALIST GROUP (GTSG) ISBN-10: 1-905164-74-2 ISBN-13: 978-1-905164-74-5 forms part of the Species Survival Commission’s network of over 7,000 volunteers working to stop the loss of plants, animals and their habitats. Reproduction of any part of the publication for SSC is the largest of the six Commissions of IUCN – The International educational, conservation and other non-profit purposes is authorized without prior permission from Union for Conservation of Nature. It serves as the main source of advice the copyright holder, provided that the source is fully to the Union and its members on the technical aspects of species acknowledged. conservation. The aims of the IUCN/SSC Global Tree Specialist Group Reproduction for resale or other commercial purposes are to promote and implement global red listing for trees and to act in is prohibited without prior written permission from the an advisory capacity to the Global Trees Campaign. copyright holder. Recommended citation: Crowley, D., Barstow, M., Rivers, M. -

Wa Shan – Emei Shan, a Further Comparison
photograph © Zhang Lin A rare view of Wa Shan almost minus its shroud of mist, viewed from the Abies fabri forested slopes of Emei Shan. At its far left the mist-filled Dadu River gorge drops to 500-600m. To its right the 3048m high peak of Mao Kou Shan climbed by Ernest Wilson on 3 July 1903. “As seen from the top of Mount Omei, it resembles a huge Noah’s Ark, broadside on, perched high up amongst the clouds” (Wilson 1913, describing Wa Shan floating in the proverbial ‘sea of clouds’). Wa Shan – Emei Shan, a further comparison CHRIS CALLAGHAN of the Australian Bicentennial Arboretum 72 updates his woody plants comparison of Wa Shan and its sister mountain, World Heritage-listed Emei Shan, finding Wa Shan to be deserving of recognition as one of the planet’s top hotspots for biological diversity. The founding fathers of modern day botany in China all trained at western institutions in Europe and America during the early decades of last century. In particular, a number of these eminent Chinese botanists, Qian Songshu (Prof. S. S. Chien), Hu Xiansu (Dr H. H. Hu of Metasequoia fame), Chen Huanyong (Prof. W. Y. Chun, lead author of Cathaya argyrophylla), Zhong Xinxuan (Prof. H. H. Chung) and Prof. Yung Chen, undertook their training at various institutions at Harvard University between 1916 and 1926 before returning home to estab- lish the initial Chinese botanical research institutions, initiate botanical exploration and create the earliest botanical gardens of China (Li 1944). It is not too much to expect that at least some of them would have had personal encounters with Ernest ‘Chinese’ Wilson who was stationed at the Arnold Arboretum of Harvard between 1910 and 1930 for the final 20 years of his life. -

Download PCN-Acer-2017-Holdings.Pdf
PLANT COLLECTIONS NETWORK MULTI-INSTITUTIONAL ACER LIST 02/13/18 Institutional NameAccession no.Provenance* Quan Collection Id Loc.** Vouchered Plant Source Acer acuminatum Wall. ex D. Don MORRIS Acer acuminatum 1994-009 W 2 H&M 1822 1 No Quarryhill BG, Glen Ellen, CA QUARRYHILL Acer acuminatum 1993.039 W 4 H&M1822 1 Yes Acer acuminatum 1993.039 W 1 H&M1822 1 Yes Acer acuminatum 1993.039 W 1 H&M1822 1 Yes Acer acuminatum 1993.039 W 1 H&M1822 1 Yes Acer acuminatum 1993.076 W 2 H&M1858 1 No Acer acuminatum 1993.076 W 1 H&M1858 1 No Acer acuminatum 1993.139 W 1 H&M1921 1 No Acer acuminatum 1993.139 W 1 H&M1921 1 No UBCBG Acer acuminatum 1994-0490 W 1 HM.1858 0 Unk Sichuan Exp., Kew BG, Howick Arb., Quarry Hill ... Acer acuminatum 1994-0490 W 1 HM.1858 0 Unk Sichuan Exp., Kew BG, Howick Arb., Quarry Hill ... Acer acuminatum 1994-0490 W 1 HM.1858 0 Unk Sichuan Exp., Kew BG, Howick Arb., Quarry Hill ... UWBG Acer acuminatum 180-59 G 1 1 Yes National BG, Glasnevin Total of taxon 18 Acer albopurpurascens Hayata IUCN Red List Status: DD ATLANTA Acer albopurpurascens 20164176 G 1 2 No Crug Farm Nursery QUARRYHILL Acer albopurpurascens 2003.088 U 1 1 No Total of taxon 2 Acer amplum (Gee selection) DAWES Acer amplum (Gee selection) D2014-0117 G 1 1 No Gee Farms, Stockbridge, MI 49285 Total of taxon 1 Acer amplum 'Gold Coin' DAWES Acer amplum 'Gold Coin' D2015-0013 G 1 2 No Gee Farms, Stockbridge, MI 49285, USA Acer amplum 'Gold Coin' D2017-0075 G 2 2 No Shinn, Edward T., Wall Township, NJ 07719-9128 Total of taxon 3 Acer argutum Maxim. -

Biodiversity in Karnali Province: Current Status and Conservation
Biodiversity in Karnali Province: Current Status and Conservation Karnali Province Government Ministry of Industry, Tourism, Forest and Environment Surkhet, Nepal Biodiversity in Karnali Province: Current Status and Conservation Karnali Province Government Ministry of Industry, Tourism, Forest and Environment Surkhet, Nepal Copyright: © 2020 Ministry of Industry, Tourism, Forest and Environment, Karnali Province Government, Surkhet, Nepal The views expressed in this publication do not necessarily reflect those of Ministry of Tourism, Forest and Environment, Karnali Province Government, Surkhet, Nepal Editors: Krishna Prasad Acharya, PhD and Prakash K. Paudel, PhD Technical Team: Achyut Tiwari, PhD, Jiban Poudel, PhD, Kiran Thapa Magar, Yogendra Poudel, Sher Bahadur Shrestha, Rajendra Basukala, Sher Bahadur Rokaya, Himalaya Saud, Niraj Shrestha, Tejendra Rawal Production Editors: Prakash Basnet and Anju Chaudhary Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Acharya, K. P., Paudel, P. K. (2020). Biodiversity in Karnali Province: Current Status and Conservation. Ministry of Industry, Tourism, Forest and Environment, Karnali Province Government, Surkhet, Nepal Cover photograph: Tibetan wild ass in Limi valley © Tashi R. Ghale Keywords: biodiversity, conservation, Karnali province, people-wildlife nexus, biodiversity profile Editors’ Note Gyau Khola Valley, Upper Humla © Geraldine Werhahn This book “Biodiversity in Karnali Province: Current Status and Conservation”, is prepared to consolidate existing knowledge about the state of biodiversity in Karnali province. The book presents interrelated dynamics of society, physical environment, flora and fauna that have implications for biodiversity conservation. -

Plant Inventory No. 173
Plant Inventory No. 173 UNITED STATES DEPARTMENT OF AGRICULTURE Washington, D.C., March 1969 UCED JANUARY 1 to DECEMBER 31, 1965 (N( >. 303628 to 310335) MAY 2 6 1969 CONTENTS Page Inventory 8 Index of common and scientific names 257 This inventory, No. 173, lists the plant material (Nos. 303628 to 310335) received by the New Crops Research Branch, Crops Research Division, Agricultural Research Service, during the period from January 1 to December 31, 1965. The inventory is a historical record of plant material introduced for Department and other specialists and is not to be considered as a list of plant ma- terial for distribution. The species names used are those under which the plant ma- terial was received. These have been corrected only for spelling, authorities, and obvious synonymy. Questions related to the names published in the inventory and obvious errors should be directed to the author. If misidentification is apparent, please submit an herbarium specimen with flowers and fruit for reidentification. HOWARD L. HYLAND Botanist Plant Industry Station Beltsville, Md. INVENTORY 303628. DIGITARIA DIDACTYLA Willd. var DECALVATA Henr. Gramineae. From Australia. Plants presented by the Commonwealth Scientific and In- dustrial Research Organization, Canberra. Received Jan. 8, 1965. Grown at West Ryde, Sydney. 303629. BRASSICA OLERACEA var. CAPITATA L. Cruciferae. Cabbage. From the Republic of South Africa. Seeds presented by Chief, Division of Plant and Seed Control, Department of Agricultural Technical Services, Pretoria. Received Jan. 11, 1965. Cabbage Number 20. 303630 to 303634. TRITICUM AESTIVUM L. Gramineae. From Australia. Seeds presented by the Agricultural College, Roseworthy. Received Jan. 11,1965. -

September 14, 2005
FOR INFORMATION DA-2005-28 September 14, 2005 SUBJECT: Phytophthora ramorum (sudden oak death, ramorum blight & die back): Revision of Associated Regulated Articles (nursery stock); Additions to APHIS List of Hosts and Plants Associated with Phytophthora ramorum TO: STATE AND TERRITORY AGRICULTURAL REGULATORY OFFICIALS On February 14, 2002, APHIS published an interim rule in the Federal Register for Phytophthora ramorum (7 CFR 301.92). This rule restricts the movement of certain restricted and regulated articles to prevent the artificial spread interstate of this disease-causing organism from areas where the disease is established. We also issued an Emergency Federal Order dated December 21, 2004, to regulate certain nurseries and plants to prevent the spread of the pathogen through nursery plants from California, Oregon and Washington. We have now learned that certain additional plants require regulating in order to control the artificial spread of this disease. The purpose of this SPRO is to provide notification that APHIS is listing and thus regulating eight new plants. We received information from the Department of Environment, Forestry, and Rural Affairs (DEFRA) of the United Kingdom that they had officially identified three new plants associated with P. ramorum. These are: Acer laevigatum - Aceraceae (Evergreen maple), Michelia doltsopa - Magnoliaceae (Michelia), Quercus petraea - Fagaceae (Sessile oak). These three were found in green areas (a public garden or park-like setting). A California researcher has alerted us that five plants found in the infested area of California demonstrated symptoms and have been determined to be infected with P. ramorum. These plants are: Adiantum aleuticum – Polypodiaceae (Western maidenhair fern), Fraxinus latifolia – Oleaceae (Oregon ash), Osmorhiza berteroi – Apiaceae (Sweet Cicely), Torreya californica – Taxaceae (California nutmeg), and Vancouveria planipetala – Berberidaceae (Redwood ivy). -

Background India: General Information
Important BIrd and BIodIVErSItY arEaS In IndIa – bACKGROUnd BACKGROUND OTTO PFISTER OTTO More than 1,200 species of birds are found in India, including some spectacular species such as the Bar-headed Goose Anser indicus INDIA: GENERAL INFORMATION ndia is situated between latitudes 8° 4’ and 37° N, and (October–March). However, in south India, the winter is Ilongitudes 68° 7’ and 97° 25’ E, and is bounded on the not as cold as in north India. It is marked by clear skies, southwest by the Arabian Sea and on the southeast by the hot days, and cool nights. This kind of weather prevails Bay of Bengal. To the north and northeast lies the mighty from September to March. The southwest monsoon sets Himalayan range. To the west lies Pakistan and to the in over Kerala in June, progresses towards the north and east, Bangladesh and Myanmar. In the north, Tibet, China, envelops the entire country by the end of July. The eastern Nepal, and Bhutan share international boundaries with coastal regions – the coasts of Andhra Pradesh and Tamil India. To the south Sri Lanka shares the maritime boundary Nadu – experience the northeast monsoon between October and is separated from India by a narrow channel of the Bay and November. Along the east coast, this period is marked of Bengal formed by the Palk Strait and the Gulf of Mannar by cyclones due to severe atmospheric depressions in the (Mathew 2003). Bay of Bengal and the Indian Ocean that move towards the India is one of the largest countries of the world and mainland at a high speed, causing widespread destruction covers an area of about 3,287,263 sq. -
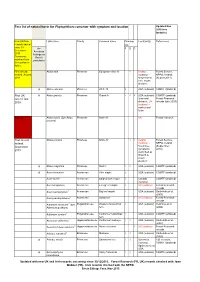
Fera List of Natural Hosts for Phytophthora Ramorum with Symptom and Location Updated Nov 2015 (See Footnote)
Fera list of natural hosts for Phytophthora ramorum with symptom and location Updated Nov 2015 (see footnote) New UK/Irish Latin name Family Common name Damage Location(s) References records added type* since 15 A= F D C December American 2010. findings no Comments Koch's retained from postulates last update in italics. First record. Abies alba Pinaceae European silver fir √ Ireland Forest Service, Ireland, August (outdoor) - NPPO, Ireland, 2011 single forest (August 2011). tree, crown dieback. A Abies concolor Pinaceae White fir √ USA (outdoor) COMTF (undated) First UK A Abies grandis Pinaceae Grand fir √ √ √ USA (outdoor) - COMTF (undated) record, late foliar and Forest Research 2009. dieback; UK records (late 2009) (outdoor) - canker and foliar Nov-15 Abies noblis (Syn Abies Pinaceae Noble fir UK Forest research procera) First record, Abies procera Pinaceae Noble fir √ √ Ireland Forest Service, Ireland, (outdoor) - NPPO, Ireland September forest tree (September, 2010 symptoms 2010). described as 'branch & crown dieback'. A Abies magnifica Pinaceae Red fir √ √ USA (outdoor) COMTF (undated) A Acer circinatum Aceraceae Vine maple √ USA (outdoor) COMTF (undated) Acer davidii Aceraceae Striped bark maple √ Canada COMTF (undated) (nursery) Acer laevigatum Aceraceae Evergreen maple √ UK (outdoor) Forest Research records Acer macrophyllum 1 Aceraceae Big leaf maple √ USA (outdoor) Garbelotto et al . (2003) Acer pseudoplatanus 1 Aceraceae Sycamore √ UK (outdoor) Forest Research records Adiantum aleuticum 1 [syn. Polypoidiaceae Western maidenhair √ USA (outdoor) Vettraino et al. Adiantum pedatum] fern (2006) Adiantum jordanii 1 Polypoidiaceae California maidenhair √ USA (outdoor) COMTF (undated) fern Aesculus californica 1 Hippocastanaceae Californian buckeye √ √ USA (outdoor) Garbelotto et al. (2003) Aesculus hippocastanum 1 Hippocastanaceae Horse chestnut √ UK (outdoor) Forest Research records Arbutus menziesii 1 Ericaceae Madrone √ √ USA (outdoor) Garbelotto et al.