Assessing Freshwater Mussels (Bivalvia: Unionidae) in South Dakota and Identifying Drivers of Assemblage Variation Kaylee L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2020-2021 Regulations Book of Game, Fish, Furbearers, and Other Wildlife
ALABAMA REGULATIONS 2020-2021 GAME, FISH, FURBEARERS, AND OTHER WILDLIFE REGULATIONS RELATING TO GAME, FISH, FURBEARERS AND OTHER WILDLIFE KAY IVEY Governor CHRISTOPHER M. BLANKENSHIP Commissioner EDWARD F. POOLOS Deputy Commissioner CHUCK SYKES Director FRED R. HARDERS Assistant Director The Department of Conservation and Natural Resources does not discriminate on the basis of race, color, religion, age, sex, national origin, disability, pregnancy, genetic information or veteran status in its hiring or employment practices nor in admission to, access to, or operations of its programs, services or activities. This publication is available in alternative formats upon request. O.E.O. U.S. Department of the Interior Washington, D.C. 20204 TABLE OF CONTENTS Division of Wildlife and Freshwater Fisheries Personnel: • Administrative Office .......................................... 1 • Aquatic Education ................................................ 9 • Carbon Hill Fish Hatchery ................................... 8 • Eastaboga Fish Hatchery ...................................... 8 • Federal Game Agents ............................................ 6 • Fisheries Section ................................................... 7 • Fisheries Development ......................................... 9 • Hunter Education .................................................. 5 • Law Enforcement Section ..................................... 2 • Marion Fish Hatchery ........................................... 8 • Mussel Management ............................................ -

Where Did Zebra Mussels Come From?
Zebra Mussels What are zebra mussels? Adult zebra mussels are small, freshwater mollusks (e.g.: clam-like organisms) named for their typical striped pattern on their shells. They are not native to Manitoba or North America. Adults have a shell and can range in size of a sesame seed and can grow up to 25 mm (1 in) long. Adult zebra mussel colour patterns can vary to the point of having only dark or light coloured shells and no stripes. Unlike our native mussels, zebra mussels attach themselves to solid surfaces, aquatic vegetation or each other with their byssal threads (Figure 1). Figure 1. A cluster of adult zebra mussels attached to a rock. Photo credit: Manitoba Sustainable Development. Larval zebra mussels are called veligers. They are so small they cannot be seen with the naked eye. Veligers are free-floating in the water and do not attach to hard surfaces until they reach the juvenile or shelled stage. Veligers do not swim on their own they move primarily by water movement. They can also be transported in undrained water found in watercraft, motors, and compartments or in water-related equipment such as bait buckets. Where did zebra mussels come from? Zebra mussels are native to Eastern Europe and Western Russia. They were brought over to the Great Lakes in ballast water of ocean-going freighters. The ballast water containing aquatic invasive species such as zebra mussels was dumped into the Great Lakes. From there zebra mussels have been moved by human activity and have greatly expanded their range. What are their breeding habits and life cycle? Zebra mussels are prolific breeders. -

Fishing the Red River of the North
FISHING THE RED RIVER OF THE NORTH The Red River boasts more than 70 species of fish. Channel catfish in the Red River can attain weights of more than 30 pounds, walleye as big as 13 pounds, and northern pike can grow as long as 45 inches. Includes access maps, fishing tips, local tourism contacts and more. TABLE OF CONTENTS YOUR GUIDE TO FISHING THE RED RIVER OF THE NORTH 3 FISHERIES MANAGEMENT 4 RIVER STEWARDSHIP 4 FISH OF THE RED RIVER 5 PUBLIC ACCESS MAP 6 PUBLIC ACCESS CHART 7 AREA MAPS 8 FISHING THE RED 9 TIP AND RAP 9 EATING FISH FROM THE RED RIVER 11 CATCH-AND-RELEASE 11 FISH RECIPES 11 LOCAL TOURISM CONTACTS 12 BE AWARE OF THE DANGERS OF DAMS 12 ©2017, State of Minnesota, Department of Natural Resources FAW-471-17 The Minnesota DNR prohibits discrimination in its programs and services based on race, color, creed, religion, national origin, sex, public assistance status, age, sexual orientation or disability. Persons with disabilities may request reasonable modifications to access or participate in DNR programs and services by contacting the DNR ADA Title II Coordinator at [email protected] or 651-259-5488. Discrimination inquiries should be sent to Minnesota DNR, 500 Lafayette Road, St. Paul, MN 55155-4049; or Office of Civil Rights, U.S. Department of the Interior, 1849 C. Street NW, Washington, D.C. 20240. This brochure was produced by the Minnesota Department of Natural Resources, Division of Fish and Wildlife with technical assistance provided by the North Dakota Department of Game and Fish. -

Biology of the Freshwater Drum in Western Lake Erie
THE OHIO JOURNAL OF SCIENCE Vol. 67 NOVEMBER, 1967 No. 6 BIOLOGY OF THE FRESHWATER DRUM IN WESTERN LAKE ERIE THOMAS A. EDSALL U.S. Department of the Interior, Bureau of Commercial Fisheries, Biological Laboratory, A nn A rbor, Michigan ABSTRACT Information on the biology of the freshwater drum or sheepshead (Aplodinotus grun- niens) was collected in Lake Erie during a fishery and limnological study made by the Bureau of Commerical Fisheries in 1957 and 1958. Growth of the sheepshead in 1958 was slower than in 1927, and slower than the growth in most other waters. Males and females grew at the same rate through the 4th year of life, but thereafter the females grew faster. Males required more than 13 years and females 11 years to reach 17 inches. A weight of 2 pounds was attained in the 12th year of life by males and in the 10th year by females. Annulus formation extended from mid-June to early August for age-groups I-IV (2nd through 5th year of life). Younger fish started growth earlier in the season than the older fish, and the larger, faster growing members of an age group began growth earlier than the smaller fish. The growing season in 1958 ended in early October. Bottom-water temperatures were about 65°P when growth started (mid-June) and 58°F when growth ended. Growth was most rapid in August when temperatures were highest for the year (72 °F). Growth of young of the year, but not that of older fish, was positively correlated with temperature during the 1951-57 growing seasons. -
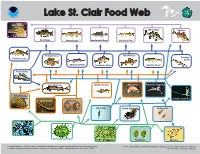
Lake St. Clair Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake St. Clair Food Web MENT OF C Sea Lamprey White Bass Walleye Rock Bass Muskellunge Smallmouth Bass Northern Pike Pumpkinseed Yellow Perch Rainbow Common Carp Smelt Channel Catfish Freshwater Drum Spottail Shiner Round Goby Lake Sturgeon Mayfly nymphs Gammarus Raptorial waterflea Zebra/Quagga mussels Invasive waterflea Chironomids Mollusks Calanoids Native waterflea Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake St. Clair, 2009. 734-741-2235 - www.glerl.noaa.gov Lake St. Clair Food Web Sea Lamprey Planktivores/Benthivores Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Lake Sturgeon (Acipenser fulvscens). Endangered over most of its historic fastens onto its prey and rasps out a hole with its rough tongue. range. Its diet commonly includes small clams, snails, crayfish, sideswimmers, aquatic insect larvae, algae, and other plant matter. Piscivores (Fish Eaters) Macroinvertebrates White bass (Morone Chrysops). Prefers clear open water in lakes and large rivers. Chironomids/Oligochaetes. Larval insects and worms that live on the lake Visual feeders, uses sight instead of smell to find prey. -

Long-Term Changes in Population Statistics of Freshwater Drum (Aplodinotus Grunniens) in Lake Winnebago, Wisconsin, Using Otolit
LONG-TERM CHANGES IN POPULATION STATISTICS OF FRESHWATER DRUM ( APLODINOTUS GRUNNIENS ) IN LAKE WINNEBAGO, WISCONSIN, USING OTOLITH GROWTH CHRONOLOGIES AND BOMB RADIOCARBON AGE VALIDATION by Shannon L. Davis-Foust A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biological Sciences At The University of Wisconsin-Milwaukee August 2012 LONG-TERM CHANGES IN POPULATION STATISTICS OF FRESHWATER DRUM ( APLODINOTUS GRUNNIENS ) IN LAKE WINNEBAGO, WISCONSIN, USING OTOLITH GROWTH CHRONOLOGIES AND BOMB RADIOCARBON AGE VALIDATION by Shannon L. Davis-Foust A Dissertation Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biological Sciences at The University of Wisconsin-Milwaukee August 2012 Major Professor Dr. Rebecca Klaper Date Graduate School Approval Date ii ABSTRACT LONG-TERM CHANGES IN POPULATION STATISTICS OF FRESHWATER DRUM ( APLODINOTUS GRUNNIENS ) IN LAKE WINNEBAGO, WISCONSIN, USING OTOLITH GROWTH CHRONOLOGIES AND BOMB RADIOCARBON AGE VALIDATION by Shannon Davis-Foust The University of Wisconsin-Milwaukee, 2012 Under the Supervision of Dr. Rebecca Klaper Estimating fish population statistics such as mortality and survival requires the use of fish age data, so it is important that age determinations are accurate. Scales have traditionally, and erroneously, been used to determine age in freshwater drum (Aplodinotus grunniens ) in the majority of past studies. I used bomb radiocarbon dating to validate that sagittal otoliths of drum are the only accurate structure (versus spines or scales) for age determinations. Drum grow slower and live longer than previously recognized by scale reading. Drum otoliths can be used not only to determine age, but also to estimate body length. -

Making a Big Splash with Louisiana Fishes
Making a Big Splash with Louisiana Fishes Written and Designed by Prosanta Chakrabarty, Ph.D., Sophie Warny, Ph.D., and Valerie Derouen LSU Museum of Natural Science To those young people still discovering their love of nature... Note to parents, teachers, instructors, activity coordinators and to all the fishermen in us: This book is a companion piece to Making a Big Splash with Louisiana Fishes, an exhibit at Louisiana State Universi- ty’s Museum of Natural Science (MNS). Located in Foster Hall on the main campus of LSU, this exhibit created in 2012 contains many of the elements discussed in this book. The MNS exhibit hall is open weekdays, from 8 am to 4 pm, when the LSU campus is open. The MNS visits are free of charge, but call our main office at 225-578-2855 to schedule a visit if your group includes 10 or more students. Of course the book can also be enjoyed on its own and we hope that you will enjoy it on your own or with your children or students. The book and exhibit was funded by the Louisiana Board Of Regents, Traditional Enhancement Grant - Education: Mak- ing a Big Splash with Louisiana Fishes: A Three-tiered Education Program and Museum Exhibit. Funding was obtained by LSUMNS Curators’ Sophie Warny and Prosanta Chakrabarty who designed the exhibit with Southwest Museum Services who built it in 2012. The oarfish in the exhibit was created by Carolyn Thome of the Smithsonian, and images exhibited here are from Curator Chakrabarty unless noted elsewhere (see Appendix II). -

Iowa Fishing Regulations
www.iowadnr.gov/fishing 1 Contents What’s New? Be a Responsible Angler .....................................3 • Mississippi River walleye length limit License & Permit Requirements ..........................3 changes - length limits in Mississippi Threatened & Endangered Species ....................4 River Pools 12-20 now include the entire Health Benefits of Eating Fish .............................4 Mississippi River in Iowa (p. 12). General Fishing Regulations ...............................5 • Missouri River paddlefish season start Fishing Seasons & Limits ....................................9 date changed to Feb. 1 (p. 11) Fish Identification...............................................14 • Virtual fishing tournaments added to License Agreements with Bordering States .......16 Iowa DNR special events applications Health Advisories for Eating Fish.......................17 - the definition of fishing tournaments now Aquatic Invasive Species...................................18 includes virtual fishing tournaments (p. 6) Fisheries Offices Phone Numbers .....................20 First Fish & Master Angler Awards ....................21 Conservation Officers Phone Numbers .............23 License and Permit Fees License/Permit Resident Nonresident On Sale Dec. 15, 2020 On Sale Jan. 1, 2021 Annual 16 years old and older $22.00 $48.00 3-Year $62.00 Not Available 7-Day $15.50 $37.50 3-Day Not Available $20.50 1-Day $10.50 $12.00 Annual Third Line Fishing Permit $14.00 $14.00 Trout Fee $14.50 $17.50 Lifetime (65 years old and older) $61.50 Not Available Boundary Water Sport Trotline $26.00 $49.50 Fishing Tournament Permit $25.00 $25.00 Fishing, Hunting, Habitat Fee Combo $55.00 Not Available Paddlefish Fishing License & Tag $25.50 $49.00 Give your kids a lifetime of BIG memories The COVID-19 pandemic ignited Iowans’ pent-up passion to get out and enjoy the outdoors. -

Upper Minnesota River Watershed Five Year Strategic Plan
UPPER MINNESOTA RIVER WATERSHED FIVE YEAR STRATEGIC PLAN In Cooperation With: East Dakota Water Development District South Dakota Conservation Districts South Dakota Association of Conservation Districts South Dakota Department of Environment and Natural Resources USDA Natural Resources Conservation Service Date: August 2012 Prepared by: TABLE OF CONTENTS Executive Summary ...........................................................................................................6 Introduction ........................................................................................................................8 1.1 Project Background and Scope ........................................................................8 1.2 Upper Minnesota River Watershed History ...................................................10 1.3 Upper Minnesota River Watershed Water Quality Studies ..........................13 1.4 Goals of the Upper Minnesota River Basin Project .......................................15 2.0 Causes and Sources of Impairment .......................................................................15 2.0.1 Geography, Soils, and Land Use....................................................................15 2.0.2 Water Bodies Studies and Current Status .....................................................24 2.1.0 Description of the Impairments for 303(d) Water Body Listings in the Upper Minnesota River Basin ............................................................29 2.1.1 Temperature ...................................................................................................29 -

How to Use This Checklist
How To Use This Checklist JAWLESS FISHES: SUPERCLASS AGNATHA Pikes: Family Esocidae LAMPREYS: CLASS CEPHALASPIDOMORPHI __ Grass Pickerel Esox americanus O Lampreys: Family Petromyzontidae __ Northern Pike Esox lucius O; Lake Erie and Hinckley Lake; The information presented in this checklist reflects our __ Silver Lamprey Ichthyomyzon unicuspis h current understanding of the status of fishes within Cleveland formerly common __ American brook Lamprey Lampetra appendix R; __ Muskellunge Esox masquinongy R; Lake Erie; Metroparks. You can add to our understanding by being a Chagrin River formerly common knowledgeable observer. Record your observations and __ *Sea Lamprey Petromyzon marinus O; Lake Erie; Minnows: Family Cyprinidae contact a naturalist if you find something that may be of formerly common; native to Atlantic Coast __ Golden Shiner Notemigonus crysoleucas C interest. JAWED FISHES: SUPERCLASS __ Redside Dace Clinostomus elongatus O GNATHOSTOMATA __ Southern Redbelly Dace Phoxinus erythrogaster R Species are listed taxonomically. Each species is listed with a BONY FISHES: CLASS OSTEICHTHYES __ Creek Chub Semotilus atromaculatus C common name, a scientific name and a note about its Sturgeons: Family Acipenseridae __ Hornyhead Chub Nocomis biguttatus h; formerly in occurrence within Cleveland Metroparks. Check off species __ Lake Sturgeon Acipenser fulvescens h; Lake Erie; Cuyahoga and Chagrin River drainages. that you identify in Cleveland Metroparks. Ohio endangered __ River Chub Nocomis micropogon C; Chagrin River; O Gars: Family -
![Quick ID Features for Bait Fish [Pdf]](https://docslib.b-cdn.net/cover/1607/quick-id-features-for-bait-fish-pdf-1741607.webp)
Quick ID Features for Bait Fish [Pdf]
OHIO DEPARTMENT OF NATURAL RESOURCES DIVISION OF WILDLIFE Quick ID Features for Baitfish DEALER EDITION PUB 5487-D Quick ID Features for Baitfish TABLE OF CONTENTS Common Bait Fish-At a Glance ................................03 Sliver Carp and Bighead Carp ..................................18 Common Minnows: Family Cyprinidae .....................04 Grass Carp and Black Carp ......................................19 Suckers: Family Catostomidae .................................05 Silver Carp, Bighead Carp, and Golden Shiner ............20 Gizzard Shad: Family Clupeidae ..............................06 Silver Carp, Bighead Carp, Mooneye, and Goldeye .......21 Skipjack Herring: Family Clupeidae .........................07 Silver Carp, Bighead Carp, and Skipjack Herring ..........22 Smelt (Rainbow): Family Osmeridae ........................08 Silver Carp, Bighead Carp, and Gizzard Shad ..............23 Brook Silverside: Family Atherinidae .........................09 Bowfin, Burbot, and Snakehead ...............................24 Brook Stickleback: Family Gasterosteidae .................10 Blackstripe Topminnow and Northern Studfish ..........25 Trout-Perch: Family Percopsidae .............................11 Mottled Sculpin, Tubenose Goby, and Round Goby ....26 Sculpins: Family Cottidae .......................................12 Yellow Perch, White Bass, and Eurasian Ruffe .............27 Darters: Family Percidae ........................................13 White Bass, White Perch, and Freshwater Drum ..........28 Blackstripe Topminnow: Family -

Walleye Red Lakes, Minnesota
AND Walleye Sander vitreus © Monterey Bay Aquarium Red Lakes, Minnesota Handline and Bottom Gillnet Fisheries Standard Version F3.1 November 16, 2016 The Safina Center Seafood Analysts Disclaimer: Seafood Watch and The Safina Center strive to ensure that all our Seafood Reports and recommendations contained therein are accurate and reflect the most up-to-date evidence available at the time of publication. All our reports are peer-reviewed for accuracy and completeness by external scientists with expertise in ecology, fisheries science or aquaculture. Scientific review, however, does not constitute an endorsement of the Seafood Watch program or of The Safina Center or their recommendations on the part of the reviewing scientists. Seafood Watch and The Safina Center are solely responsible for the conclusions reached in this report. We always welcome additional or updated data that can be used for the next revision. About The Safina Center The Safina Center (formerly Blue Ocean Institute) translates scientific information into language people can understand and serves as a unique voice of hope, guidance, and encouragement. The Safina Center (TSC) works through science, art, and literature to inspire solutions and a deeper connection with nature, especially the sea. Our mission is to inspire more people to actively engage as well-informed and highly motivated constituents for conservation. Led by conservation pioneer and MacArthur fellow, Dr. Carl Safina, we show how nature, community, the economy and prospects for peace are all intertwined. Through Safina’s books, essays, public speaking, PBS television series, our Fellows program and Sustainable Seafood program, we seek to inspire people to make better choices.