Long-Term Changes in Population Statistics of Freshwater Drum (Aplodinotus Grunniens) in Lake Winnebago, Wisconsin, Using Otolit
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Periwinkle Fishery of Tasmania: Supporting Management and a Profitable Industry
Periwinkle Fishery of Tasmania: Supporting Management and a Profitable Industry J.P. Keane, J.M. Lyle, C. Mundy, K. Hartmann August 2014 FRDC Project No 2011/024 © 2014 Fisheries Research and Development Corporation. All rights reserved. ISBN 978-1-86295-757-2 Periwinkle Fishery of Tasmania: Supporting Management and a Profitable Industry FRDC Project No 2011/024 June 2014 Ownership of Intellectual property rights Unless otherwise noted, copyright (and any other intellectual property rights, if any) in this publication is owned by the Fisheries Research and Development Corporation the Institute for Marine and Antarctic Studies. This publication (and any information sourced from it) should be attributed to Keane, J.P., Lyle, J., Mundy, C. and Hartmann, K. Institute for Marine and Antarctic Studies, 2014, Periwinkle Fishery of Tasmania: Supporting Management and a Profitable Industry, Hobart, August. CC BY 3.0 Creative Commons licence All material in this publication is licensed under a Creative Commons Attribution 3.0 Australia Licence, save for content supplied by third parties, logods and the Commonwealth Coat of Arms. Creative Commons Attribution 3.0 Australia Licence is a standard form licence agreement that allows you to copy, distribute, transmit and adapt this publication provided you attribute the work. A summary of the licence terms is available from creativecommons.org/licenses/by/3.0/au/deed.en. The full licence terms are available from creativecommons.org/licenses/by/3.0/au/legalcode. Inquiries regarding the licence and any use of this document should be sent to: [email protected]. Disclaimerd The authors do not warrant that the information in this document is free from errors or omissions. -

2020-2021 Regulations Book of Game, Fish, Furbearers, and Other Wildlife
ALABAMA REGULATIONS 2020-2021 GAME, FISH, FURBEARERS, AND OTHER WILDLIFE REGULATIONS RELATING TO GAME, FISH, FURBEARERS AND OTHER WILDLIFE KAY IVEY Governor CHRISTOPHER M. BLANKENSHIP Commissioner EDWARD F. POOLOS Deputy Commissioner CHUCK SYKES Director FRED R. HARDERS Assistant Director The Department of Conservation and Natural Resources does not discriminate on the basis of race, color, religion, age, sex, national origin, disability, pregnancy, genetic information or veteran status in its hiring or employment practices nor in admission to, access to, or operations of its programs, services or activities. This publication is available in alternative formats upon request. O.E.O. U.S. Department of the Interior Washington, D.C. 20204 TABLE OF CONTENTS Division of Wildlife and Freshwater Fisheries Personnel: • Administrative Office .......................................... 1 • Aquatic Education ................................................ 9 • Carbon Hill Fish Hatchery ................................... 8 • Eastaboga Fish Hatchery ...................................... 8 • Federal Game Agents ............................................ 6 • Fisheries Section ................................................... 7 • Fisheries Development ......................................... 9 • Hunter Education .................................................. 5 • Law Enforcement Section ..................................... 2 • Marion Fish Hatchery ........................................... 8 • Mussel Management ............................................ -

WMSDB - Worldwide Mollusc Species Data Base
WMSDB - Worldwide Mollusc Species Data Base Family: TURBINIDAE Author: Claudio Galli - [email protected] (updated 07/set/2015) Class: GASTROPODA --- Clade: VETIGASTROPODA-TROCHOIDEA ------ Family: TURBINIDAE Rafinesque, 1815 (Sea) - Alphabetic order - when first name is in bold the species has images Taxa=681, Genus=26, Subgenus=17, Species=203, Subspecies=23, Synonyms=411, Images=168 abyssorum , Bolma henica abyssorum M.M. Schepman, 1908 aculeata , Guildfordia aculeata S. Kosuge, 1979 aculeatus , Turbo aculeatus T. Allan, 1818 - syn of: Epitonium muricatum (A. Risso, 1826) acutangulus, Turbo acutangulus C. Linnaeus, 1758 acutus , Turbo acutus E. Donovan, 1804 - syn of: Turbonilla acuta (E. Donovan, 1804) aegyptius , Turbo aegyptius J.F. Gmelin, 1791 - syn of: Rubritrochus declivis (P. Forsskål in C. Niebuhr, 1775) aereus , Turbo aereus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) aethiops , Turbo aethiops J.F. Gmelin, 1791 - syn of: Diloma aethiops (J.F. Gmelin, 1791) agonistes , Turbo agonistes W.H. Dall & W.H. Ochsner, 1928 - syn of: Turbo scitulus (W.H. Dall, 1919) albidus , Turbo albidus F. Kanmacher, 1798 - syn of: Graphis albida (F. Kanmacher, 1798) albocinctus , Turbo albocinctus J.H.F. Link, 1807 - syn of: Littorina saxatilis (A.G. Olivi, 1792) albofasciatus , Turbo albofasciatus L. Bozzetti, 1994 albofasciatus , Marmarostoma albofasciatus L. Bozzetti, 1994 - syn of: Turbo albofasciatus L. Bozzetti, 1994 albulus , Turbo albulus O. Fabricius, 1780 - syn of: Menestho albula (O. Fabricius, 1780) albus , Turbo albus J. Adams, 1797 - syn of: Rissoa parva (E.M. Da Costa, 1778) albus, Turbo albus T. Pennant, 1777 amabilis , Turbo amabilis H. Ozaki, 1954 - syn of: Bolma guttata (A. Adams, 1863) americanum , Lithopoma americanum (J.F. -

Where Did Zebra Mussels Come From?
Zebra Mussels What are zebra mussels? Adult zebra mussels are small, freshwater mollusks (e.g.: clam-like organisms) named for their typical striped pattern on their shells. They are not native to Manitoba or North America. Adults have a shell and can range in size of a sesame seed and can grow up to 25 mm (1 in) long. Adult zebra mussel colour patterns can vary to the point of having only dark or light coloured shells and no stripes. Unlike our native mussels, zebra mussels attach themselves to solid surfaces, aquatic vegetation or each other with their byssal threads (Figure 1). Figure 1. A cluster of adult zebra mussels attached to a rock. Photo credit: Manitoba Sustainable Development. Larval zebra mussels are called veligers. They are so small they cannot be seen with the naked eye. Veligers are free-floating in the water and do not attach to hard surfaces until they reach the juvenile or shelled stage. Veligers do not swim on their own they move primarily by water movement. They can also be transported in undrained water found in watercraft, motors, and compartments or in water-related equipment such as bait buckets. Where did zebra mussels come from? Zebra mussels are native to Eastern Europe and Western Russia. They were brought over to the Great Lakes in ballast water of ocean-going freighters. The ballast water containing aquatic invasive species such as zebra mussels was dumped into the Great Lakes. From there zebra mussels have been moved by human activity and have greatly expanded their range. What are their breeding habits and life cycle? Zebra mussels are prolific breeders. -

Aging Techniques & Population Dynamics of Blue Suckers (Cycleptus Elongatus) in the Lower Wabash River
Eastern Illinois University The Keep Masters Theses Student Theses & Publications Summer 2020 Aging Techniques & Population Dynamics of Blue Suckers (Cycleptus elongatus) in the Lower Wabash River Dakota S. Radford Eastern Illinois University Follow this and additional works at: https://thekeep.eiu.edu/theses Part of the Aquaculture and Fisheries Commons Recommended Citation Radford, Dakota S., "Aging Techniques & Population Dynamics of Blue Suckers (Cycleptus elongatus) in the Lower Wabash River" (2020). Masters Theses. 4806. https://thekeep.eiu.edu/theses/4806 This Dissertation/Thesis is brought to you for free and open access by the Student Theses & Publications at The Keep. It has been accepted for inclusion in Masters Theses by an authorized administrator of The Keep. For more information, please contact [email protected]. AGING TECHNIQUES & POPULATION DYNAMICS OF BLUE SUCKERS (CYCLEPTUS ELONGATUS) IN THE LOWER WABASH RIVER By Dakota S. Radford B.S. Environmental Biology Eastern Illinois University A thesis prepared for the requirements for the degree of Master of Science Department of Biological Sciences Eastern Illinois University May 2020 TABLE OF CONTENTS Thesis abstract .................................................................................................................... iii Acknowledgements ............................................................................................................ iv List of Tables .......................................................................................................................v -

Fishing the Red River of the North
FISHING THE RED RIVER OF THE NORTH The Red River boasts more than 70 species of fish. Channel catfish in the Red River can attain weights of more than 30 pounds, walleye as big as 13 pounds, and northern pike can grow as long as 45 inches. Includes access maps, fishing tips, local tourism contacts and more. TABLE OF CONTENTS YOUR GUIDE TO FISHING THE RED RIVER OF THE NORTH 3 FISHERIES MANAGEMENT 4 RIVER STEWARDSHIP 4 FISH OF THE RED RIVER 5 PUBLIC ACCESS MAP 6 PUBLIC ACCESS CHART 7 AREA MAPS 8 FISHING THE RED 9 TIP AND RAP 9 EATING FISH FROM THE RED RIVER 11 CATCH-AND-RELEASE 11 FISH RECIPES 11 LOCAL TOURISM CONTACTS 12 BE AWARE OF THE DANGERS OF DAMS 12 ©2017, State of Minnesota, Department of Natural Resources FAW-471-17 The Minnesota DNR prohibits discrimination in its programs and services based on race, color, creed, religion, national origin, sex, public assistance status, age, sexual orientation or disability. Persons with disabilities may request reasonable modifications to access or participate in DNR programs and services by contacting the DNR ADA Title II Coordinator at [email protected] or 651-259-5488. Discrimination inquiries should be sent to Minnesota DNR, 500 Lafayette Road, St. Paul, MN 55155-4049; or Office of Civil Rights, U.S. Department of the Interior, 1849 C. Street NW, Washington, D.C. 20240. This brochure was produced by the Minnesota Department of Natural Resources, Division of Fish and Wildlife with technical assistance provided by the North Dakota Department of Game and Fish. -

Chapter 101 Minnesota Statutes 1941
MINNESOTA STATUTES 1941 101.01 DIVISION OF GAME AND FISH; FISH 846 CHAPTER 101 DIVISION OF GAME AND FISH; FISH Sec. Sec. 101.01 Manner of taking flsh 101.21 Sale of flsh caught In certain counties; o).her 101.02 Manner of taking minnows for bait flsh not bought or sold at any time 101.03 Open season for black bass and yellow bass 101.22 Prohibited methods and equipments 101.04 Open season for trout, except lake trout; 101.23 Polluting streams hours for taking 101.24 Fish screens; removal 101.05 Fishing in trout streams 101.25 Dark houses or fish houses, when used; 101.06 Open season for lake trout licenses 101.07 Open season for pike, pickerel, and muskel- 101.26 Open season for whiteflsh, tullibees, and her lunge ring 101.08 Closed season for sturgeon, hackleback, 101.27 Open season for frogs spoonbill, or paddleflsh. 101.28 Turtles and tortoises 101.09 Open season for crappies 101.29 Fishways; construction; fishing near flshways 101.10 Fishing in boundary waters forbidden 101.11 Open season for fishing in boundary waters 101.30 Fish may be taken and sold from certain lakes 101.12 Open season for sunflsh, rock bass, and other 10.1.31 Regulations by director / varieties 101.32 Restriction 101.13 Open season for sunflsh in Goodhue county 101.34 Sections 101.30 to 101.32 supplementary 101.14 Open season for carp, dogfish, redhorse, 101.35 Disposition of dead flsh sheepshead, catfish, suckers, eelpout, garfish, 101.36 Open season for fishing in Lake of the Woods bullheads, whiteflsh, and buffaloflsh 101.37 Open season for suckers and other rough flsh 101.153 Propagation of game flsh in Lake of the Woods 101.16 When and where artificial lights may be used 101.38 Fishing from towed boats prohibited in spearing certain fish 101.39 Taking of fish in natural spawning beds 101.18 Placing carp in waters prohibited prohibited 101.19 Fishing in Minneapolis 101.40 Fish screens; permits 101.20 Limit of catch 101.01 MANNER OF TAKING FISH. -

Rough Fish”: Paradigm Shift in the Conservation of Native Fishes Andrew L
PERSPECTIVE Goodbye to “Rough Fish”: Paradigm Shift in the Conservation of Native Fishes Andrew L. Rypel | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, 1 Shields Ave, Davis, CA 95616 | University of California, Davis, Center for Watershed Sciences, Davis, CA. E-mail: [email protected] Parsa Saffarinia | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Caryn C. Vaughn | University of Oklahoma, Oklahoma Biological Survey and Department of Biology, Norman, OK Larry Nesper | University of Wisconsin–Madison, Department of Anthropology, Madison, WI Katherine O’Reilly | University of Notre Dame, Department of Biological Sciences, Notre Dame, IN Christine A. Parisek | University of California, Davis, Center for Watershed Sciences, Davis, CA | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA | The Nature Conservancy, Science Communications, Boise, ID Peter B. Moyle | University of California, Davis, Center for Watershed Sciences, Davis, CA Nann A. Fangue | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Miranda Bell- Tilcock | University of California, Davis, Center for Watershed Sciences, Davis, CA David Ayers | University of California, Davis, Center for Watershed Sciences, Davis, CA | University of California, Davis, Department of Wildlife, Fish and Conservation Biology, Davis, CA Solomon R. David | Nicholls State University, Department of Biological Sciences, Thibodaux, LA While sometimes difficult to admit, perspectives of European and white males have overwhelmingly dominated fisheries science and management in the USA. This dynamic is exemplified by bias against “rough fish”— a pejorative ascribing low- to- zero value for countless native fishes. One product of this bias is that biologists have ironically worked against conservation of diverse fishes for over a century, and these problems persist today. -

Biology of the Freshwater Drum in Western Lake Erie
THE OHIO JOURNAL OF SCIENCE Vol. 67 NOVEMBER, 1967 No. 6 BIOLOGY OF THE FRESHWATER DRUM IN WESTERN LAKE ERIE THOMAS A. EDSALL U.S. Department of the Interior, Bureau of Commercial Fisheries, Biological Laboratory, A nn A rbor, Michigan ABSTRACT Information on the biology of the freshwater drum or sheepshead (Aplodinotus grun- niens) was collected in Lake Erie during a fishery and limnological study made by the Bureau of Commerical Fisheries in 1957 and 1958. Growth of the sheepshead in 1958 was slower than in 1927, and slower than the growth in most other waters. Males and females grew at the same rate through the 4th year of life, but thereafter the females grew faster. Males required more than 13 years and females 11 years to reach 17 inches. A weight of 2 pounds was attained in the 12th year of life by males and in the 10th year by females. Annulus formation extended from mid-June to early August for age-groups I-IV (2nd through 5th year of life). Younger fish started growth earlier in the season than the older fish, and the larger, faster growing members of an age group began growth earlier than the smaller fish. The growing season in 1958 ended in early October. Bottom-water temperatures were about 65°P when growth started (mid-June) and 58°F when growth ended. Growth was most rapid in August when temperatures were highest for the year (72 °F). Growth of young of the year, but not that of older fish, was positively correlated with temperature during the 1951-57 growing seasons. -

Reproductive Biology of Two Species Congregation of Adults in Groups Clearly Ensures the of Turbinidae (Mollusca: Gastropoda)
World Journal of Fish and Marine Sciences 2 (1): 14-20, 2010 ISSN 2078-4589 © IDOSI Publications, 2010 Annual Cycle of Reproduction in Turbo brunneus, from Tuticorin South East Coast of India 1R. Ramesh, 2S. Ravichandran and 2K. Kumaravel 1Department of Zoology, Government Arts College, Salem, India 2Centre of Advanced Study in Marine Biology, Annamalai University, India Abstract: This research work mainly focus on the reproductive and spawning season of Turbo brunneus a mollusk in the south east coast of India. Random samples from Turbo brunneus were collected from littoral tidal pools in Tuticorin coast, during May 2002 to April 2003. The number of male and females in the monthly samples was counted to determine the male: female ratio in the population and chi-square test was applied to test whether the population adheres to 1:1 ratio. The overall male and female ratio is found to be 1: 0.96 indicating only a slight variation in the evenness of male and female in the population. Both sexes of T. brunneus attain sexual maturity between 23 and 27mm. The mean gonadal index (G.I) was high (21.82%) in males during May, 2002 and then it decreased gradually and reached 15.52% during October 2002, which showed the low mean GI value in males for the whole study period. While for females it was high during May 2002 (23.09%) and low during September 2002(14.83%). The GI values for both the sexes were generally low until December 2002. The limited percentage of matured oocytes which exists even after spawning indicates the high possibility for partial spawning in T. -
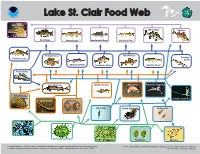
Lake St. Clair Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake St. Clair Food Web MENT OF C Sea Lamprey White Bass Walleye Rock Bass Muskellunge Smallmouth Bass Northern Pike Pumpkinseed Yellow Perch Rainbow Common Carp Smelt Channel Catfish Freshwater Drum Spottail Shiner Round Goby Lake Sturgeon Mayfly nymphs Gammarus Raptorial waterflea Zebra/Quagga mussels Invasive waterflea Chironomids Mollusks Calanoids Native waterflea Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake St. Clair, 2009. 734-741-2235 - www.glerl.noaa.gov Lake St. Clair Food Web Sea Lamprey Planktivores/Benthivores Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Lake Sturgeon (Acipenser fulvscens). Endangered over most of its historic fastens onto its prey and rasps out a hole with its rough tongue. range. Its diet commonly includes small clams, snails, crayfish, sideswimmers, aquatic insect larvae, algae, and other plant matter. Piscivores (Fish Eaters) Macroinvertebrates White bass (Morone Chrysops). Prefers clear open water in lakes and large rivers. Chironomids/Oligochaetes. Larval insects and worms that live on the lake Visual feeders, uses sight instead of smell to find prey. -

Commercial Fishing in SOUTH DAKOTA by Todd St
PAST, PRESENT AND FUTURE OF Commercial Fishing IN SOUTH DAKOTA By todd St. Sauver, GFP Fisheries program specialist If you have ever watched reality shows like Deadliest Catch, Swamp Pawn, Bottom Feeders or Alaska: Battle on the Bay, you are familiar with a few of the many types of commercial fishing practiced around the world. But did you know we have commercial fishing in South Dakota? What is commercial fishing? Put simply, it is catching fish or other aquatic animals to sell for profit. Commercial fishing operations can be small, with one person running a few hoop nets for catfish, or large, with massive trawlers and factory ships on the oceans. In the early 1800s, native game fish species were mainly valued as food sources and were commonly harvested by commercial fishermen. By the mid-1800s, these populations began to deplete, so additional sources of fish were considered. In an 1874 report by the newly-formed United States Fish Commission, Professor Spencer F. Baird suggested that the European carp (now called common carp) could solve the problem. Two years later, he again urged the Commission to consider introducing carp to the U.S. because of its ability to reproduce rapidly, grow fast, and live in waters native fish could not survive in. In 1877, the Commission made what may be the greatest fish management mistake in U.S. history. They imported 345 carp from Europe to ponds in Baltimore, Maryland. It was not long until the progeny produced by these fish were stocked into eastern lakes and rivers. Within four years, commercial fishermen were catching carp in Lake Erie and in the Illinois, Missouri and Mississippi Rivers.