Erythrocytes: Overview, Morphology, Quantity by AH Rebar Et
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Basis of Spectrin Deficiency in Beta Spectrin Durham. a Deletion
Molecular basis of spectrin deficiency in beta spectrin Durham. A deletion within beta spectrin adjacent to the ankyrin-binding site precludes spectrin attachment to the membrane in hereditary spherocytosis. H Hassoun, … , S S Chiou, J Palek J Clin Invest. 1995;96(6):2623-2629. https://doi.org/10.1172/JCI118327. Research Article We describe a spectrin variant characterized by a truncated beta chain and associated with hereditary spherocytosis. The clinical phenotype consists of a moderate hemolytic anemia with striking spherocytosis and mild spiculation of the red cells. We describe the biochemical characteristics of this truncated protein which constitutes only 10% of the total beta spectrin present on the membrane, resulting in spectrin deficiency. Analysis of reticulocyte cDNA revealed the deletion of exons 22 and 23. We show, using Southern blot analysis, that this truncation results from a 4.6-kb genomic deletion. To elucidate the basis for the decreased amount of the truncated protein on the membrane and the overall spectrin deficiency, we show that (a) the mutated gene is efficiently transcribed and its mRNA abundant in reticulocytes, (b) the mutant protein is normally synthesized in erythroid progenitor cells, (c) the stability of the mutant protein in the cytoplasm of erythroblasts parallels that of the normal beta spectrin, and (d) the abnormal protein is inefficiently incorporated into the membrane of erythroblasts. We conclude that the truncation within the beta spectrin leads to inefficient incorporation of the mutant protein into the skeleton despite its normal synthesis and stability. We postulate that this misincorporation results from conformational changes of the beta spectrin subunit affecting the binding of the abnormal heterodimer to ankyrin, and we provide evidence […] Find the latest version: https://jci.me/118327/pdf Molecular Basis of Spectrin Deficiency in p8 Spectrin Durham A Deletion within .3 Spectrin Adjacent to the Ankyrin-binding Site Precludes Spectrin Attachment to the Membrane in Hereditary Spherocytosis Hani Hassoun,* John N. -

Hereditary Spherocytosis: Clinical Features
Title Overview: Hereditary Hematological Disorders of red cell shape. Disorders Red cell Enzyme disorders Disorders of Hemoglobin Inherited bleeding disorders- platelet disorders, coagulation factor Anthea Greenway MBBS FRACP FRCPA Visiting Associate deficiencies Division of Pediatric Hematology-Oncology Duke University Health Service Inherited Thrombophilia Hereditary Disorders of red cell Disorders of red cell shape (cytoskeleton): cytoskeleton: • Mutations of 5 proteins connect cytoskeleton of red cell to red cell membrane • Hereditary Spherocytosis- sphere – Spectrin (composed of alpha, beta heterodimers) –Ankyrin • Hereditary Elliptocytosis-ellipse, elongated forms – Pallidin (band 4.2) – Band 4.1 (protein 4.1) • Hereditary Pyropoikilocytosis-bizarre red cell forms – Band 3 protein (the anion exchanger, AE1) – RhAG (the Rh-associated glycoprotein) Normal red blood cell- discoid, with membrane flexibility Hereditary Spherocytosis: Clinical features: • Most common hereditary hemolytic disorder (red cell • Neonatal jaundice- severe (phototherapy), +/- anaemia membrane) • Hemolytic anemia- moderate in 60-75% cases • Mutations of one of 5 genes (chromosome 8) for • Severe hemolytic anaemia in 5% (AR, parents ASx) cytoskeletal proteins, overall effect is spectrin • fatigue, jaundice, dark urine deficiency, severity dependant on spectrin deficiency • SplenomegalSplenomegaly • 200-300:million births, most common in Northern • Chronic complications- growth impairment, gallstones European countries • Often follows clinical course of affected -

Rbcs) in Both the Sinus and Cord, Histiocytes Werc Swollen by a Granu}Ar Substance in the Cytoplasm and Also Many Secondary Lysosomes
The UOEHAssociationUOEH Association ofofHealth Health Sciences JUOEH20(1)!11-19 (1998) 11 [Original) Pathological Study Splenomegaly Associated with Cadmium-inducedof Anemia in Rats Tetsuo HAMADA', Akihide TANIMOT02, Nobuyuki ARIMA!, Yoshihiro IDEL, Takakazu SASAGURI2, Shohei SHIMiVIRI2, Yoshitaka MURATA', Ke-Yong WANG2 and Yasuyuki SASAGURIL' 'Dopartment of'Surgical Pathotogy, University llosPital ?Dopartment qf Palhology and Cell Biotogy, Scheol of' Adlrdicine, (1itib'ersity of Occapational and Environmental Health., .lapan. }'dhatanishi-ku, Kiialp,ztshu 807-8imr, .1apan Ahstract : Splenomegaly was observcd both in male and R)malc Spraguc-Dawley rats after 1 week of exposure to CdC12 (O,6 mg (:d/kg/day), Sp]een weight reached about double that in controls by 8 weeks of Cd exposure. Histopathologica] cxamination of the enlargcd spleen rcvcaled that iron- and lipid-laden histiocytes were clustered in tha periarterial lymphatic sheath, and the red pulp appeared to be expanded. It is noteworthy that electron microscopy rcvealed markcd poikilocytosis and Hcinz body formation in red blood cells (RBCs) in both the sinus and cord, Histiocytes werc swollen by a granu}ar substance in the cytoplasm and also many secondary lysosomes. Thesc morphological findings indicatc that degradation of damagcd RBCs induced by exposure to Cd might bc promoted in the splccn and possibly cause splenomcgaly, This RBC damage-hcmolysis-splcnomegaly sequence is also considered to be associated with the etioiogy of Cd-induced anemia, In addition to the abnormal RBC degradation, nuclel of ]ymphocytes in thc Cd-cxposcd spleen exhibited high elcctron density, consistent with a preapoptotic statc suggesting the immunosupprcssive effhct ofCd. Kay woralf :spienomegaly, poiki]ocytosis, Heinz body, cadmiurn, anemia, (Received 18 November 1997, accepted I9January 1998) Introduction Cd was reported to cause anemia in dogs as early as 1896 [1], and subsequently, anemia was found in humans after Cd exposure [2]. -

Hereditary Spherocytosis
Hereditary Spherocytosis o RBC band 3 protein testing is a very sensitive and Indications for Ordering specific test for the diagnosis of hereditary Use to confirm diagnosis of hereditary spherocytosis when spherocytosis hemolytic anemia and spherocytes are present Physiology • RBC band 3 protein is a major structural protein of RBCs Test Description o Reduction in the amount of band 3 fluorescence after Test Methodology binding with EMA correlates with spherocytosis • Red blood cell (RBC) surface protein band 3 staining with Genetics eosin-5-maleimide (EMA) analyzed by flow cytometry Clinical Validation Genes: ANK1, EPB42, SLC4A1, SPTA1, SPTB • Validated against the clinical diagnosis of hereditary Inheritance spherocytosis supported by osmotic fragility and/or • Autosomal dominant: 75% molecular testing • Autosomal recessive: 25% Tests to Consider Penetrance: variable Structure/Function Primary Test • Chromosomal location: 17q21.31 RBC Band 3 Protein Reduction in Hereditary Spherocytosis • Provides structure for the red cell cytoskeleton 2008460 • Use to confirm diagnosis of hereditary spherocytosis Test Interpretation when hemolytic anemia and spherocytes are present Sensitivity/Specificity Related Test • Clinical sensitivity: 93% Osmotic Fragility, Erythrocyte 2002257 • Analytical sensitivity/specificity: unknown • Functional testing of RBC sensitivity to osmotic stress Results Disease Overview • Normal o Normal staining of band 3 protein with EMA does not Prevalence: 1/2,000 in northern Europeans suggest hereditary spherocytosis -

Hereditary Spherocytosis (HS)
Hereditary Spherocytosis (HS) Hereditary spherocytosis (HS) is a medical term for a condition What are the symptoms of HS? which affects the red blood cells. Symptoms of HS are due to 2 processes: hemolysis and anemia. What are red blood cells? When red blood cells break down (hemolysis), they release biliruin Blood contains 3 types of cells: red blood cells (RBC), white blood into the bloodstream, which causes yellowing of the skin (jaundice) cells (WBC), and platelets. Red blood cells are the most common and eyes. Hemolysis causes different problems depending on the type of blood cell, and are responsible for delivering oxygen to all age: parts of the body. • Newborns – may need light therapy or further measures Red blood cells, like white cells and platelets, are continuously made • Children – increasing size of the spleen in the bone marrow. When released into the bloodstream, the average lifespan of a red cell is 120 days. • Teens/adults – gallstones that may require surgery Under the microscope, normal red cells are shaped like discs or If enough red cells are destroyed, the red cell count will be low donuts with the centers partially scooped out. This shape makes (anemia). Symptoms of anemia include looking pale, being tired or them very soft and flexible, so they can easily squeeze through even weak, headaches, poor concentration, and challenges with behavior very small blood vessels. and school. Sometimes there is a problem with the wall of the red cell, and they How is HS treated? change shape to look like spheres or balls. These cells are called ‘spherocytes’. -

Hemolytic Anemia (Aiha) Clinical Hemolysis Indicators
October 15, 2013 GME Morning report Harmesh Naik, MD. AUTO IMMUNE HEMOLYTIC ANEMIA (AIHA) CLINICAL HEMOLYSIS INDICATORS Anemia High reticulocyte counts Elevated LDH level Unconjugated (indirect) hyper bilirubinemia Reduced haptoglobin level Hemoglobinuria COMPARISON Autoimmune hemolytic anemia Hereditary spherocytosis Hemolysis Hemolysis Spherocytes Spherocytes Splenomegaly Splenomegaly DAT positive DAT negative No Family history Family history TYPE OF ANTIBODY Auto antibody – generally seen in AIHA- detected generally by DAT Allo antibody – generally seen after transfusion of donor RBCs containing antigen – detected generally by IAT ANTI GLOBULIN TEST (COOMB’S TEST) Developed by Coombs in 1945 THE DIRECT ANTIGLOBULIN TEST (DAT) AND INDIRECT ANTIGLOBULIN TEST (IAT). Zarandona J M , and Yazer M H CMAJ 2006;174:305-307 DIRECT COOMB’S TEST (DAT) Detects IgG or complement coating on surface of circulating RBCs Detects In Vivo sensitization of RBCs Useful in differentiating immune mediated hemolysis Generally detects auto-antibodies in AIHA DIRECT COOMB’S TEST (DAT) Auto antibodies in AIHA are generally IgG antibodies with high affinity for human RBCs at 37*C 9body temperature) As a result most of antibody is bound to RBCs and very little is free in plasma So DAT can find RBC bound antibody effectively INDIRECT COOMB’S TEST (IAT OR ANTIBODY SCREENING) Detects antibodies in serum (IgG antibodies in serum) Detects in vitro sensitization of RBCs COLD AUTO ANTIBODY Spontaneous agglutination after incubation of patient serum -
Complete Blood Count in Primary Care
Complete Blood Count in Primary Care bpac nz better medicine Editorial Team bpacnz Tony Fraser 10 George Street Professor Murray Tilyard PO Box 6032, Dunedin Clinical Advisory Group phone 03 477 5418 Dr Dave Colquhoun Michele Cray free fax 0800 bpac nz Dr Rosemary Ikram www.bpac.org.nz Dr Peter Jensen Dr Cam Kyle Dr Chris Leathart Dr Lynn McBain Associate Professor Jim Reid Dr David Reith Professor Murray Tilyard Programme Development Team Noni Allison Rachael Clarke Rebecca Didham Terry Ehau Peter Ellison Dr Malcolm Kendall-Smith Dr Anne Marie Tangney Dr Trevor Walker Dr Sharyn Willis Dave Woods Report Development Team Justine Broadley Todd Gillies Lana Johnson Web Gordon Smith Design Michael Crawford Management and Administration Kaye Baldwin Tony Fraser Kyla Letman Professor Murray Tilyard Distribution Zane Lindon Lyn Thomlinson Colleen Witchall All information is intended for use by competent health care professionals and should be utilised in conjunction with © May 2008 pertinent clinical data. Contents Key points/purpose 2 Introduction 2 Background ▪ Haematopoiesis - Cell development 3 ▪ Limitations of reference ranges for the CBC 4 ▪ Borderline abnormal results must be interpreted in clinical context 4 ▪ History and clinical examination 4 White Cells ▪ Neutrophils 5 ▪ Lymphocytes 9 ▪ Monocytes 11 ▪ Basophils 12 ▪ Eosinophils 12 ▪ Platelets 13 Haemoglobin and red cell indices ▪ Low haemoglobin 15 ▪ Microcytic anaemia 15 ▪ Normocytic anaemia 16 ▪ Macrocytic anaemia 17 ▪ High haemoglobin 17 ▪ Other red cell indices 18 Summary Table 19 Glossary 20 This resource is a consensus document, developed with haematology and general practice input. We would like to thank: Dr Liam Fernyhough, Haematologist, Canterbury Health Laboratories Dr Chris Leathart, GP, Christchurch Dr Edward Theakston, Haematologist, Diagnostic Medlab Ltd We would like to acknowledge their advice, expertise and valuable feedback on this document. -

Red Blood Cell Morphology in Patients with Β-Thalassemia Minor
J Lab Med 2017; 41(1): 49–52 Short Communication Carolin Körber, Albert Wölfler, Manfred Neubauer and Christoph Robier* Red blood cell morphology in patients with β-thalassemia minor DOI 10.1515/labmed-2016-0052 Keywords: β-thalassemia minor; erythrocytes; red blood Received July 11, 2016; accepted October 20, 2016; previously published cells; red blood cell morphology. online December 10, 2016 Abstract In β-thalassemias, the examination of a peripheral blood (PB) smear may provide relevant clues to initial diagnosis. Background: A systematic analysis of the occurrence of Complete laboratory investigation consists of the determina- red blood cell (RBC) abnormalities in β-thalassemia minor tion of the complete blood count, assessment of red blood has not been performed to date. This study aimed to iden- cell (RBC) morphology, high performance liquid chroma- tify and quantify the frequency of RBC abnormalities in tography (HPLC), hemoglobin electrophoresis and, where patients with β-thalassemia minor. necessary, DNA analysis [1]. Especially in the clinically Methods: We examined blood smears of 33 patients with severe forms referred to as β-thalassemia major and interme- β-thalassemia minor by light microscopy for the occur- dia, RBC abnormalities are often markedly apparent [2]. In rence of 15 defined RBC abnormalities. In the case of posi- β-thalassemia minor, also called β-thalassemia trait, the car- tivity, the abnormal cells/20 high power fields (HPF) at riers are usually clinically asymptomatic, showing persistent 1000-fold magnification were counted. microcytosis and hypochromia or mild microcytic anemia [1, Results: Anisocytosis, poikilocytosis and target cells 3]. The PB smear may show microcytosis, hypochromia and, (median 42/20 HPF) were observed in all, and ovalocytes infrequently, poikilocytosis [2]. -

Hereditary Spherocytosis (HS)
CP Presentation Rania Abadeer, MD HPI • 20-year-old white college student, diagnosed with infectious mononucleosis 6 months ago • He recovered, but a follow-up CBC revealed a hemoglobin of 9.3 gm/dl and a reticulocytosis of 7.4% • A direct Coombs test (direct anti- globulin test) was negative, and liver function tests normal except for an indirect bilirubin at 2.5 mg/dl Physical Exam • He is a healthy young man who has minimal scleral icterus • Non-tender liver with a vertical span of 10 cm, and a soft spleen tip which descended 4 cm below the LCM with deep inspiration Labs • WBC 6400 • Platelets 195,000 • Red blood cell indicies – Hgb 10.8 – Hct 30 – RBC 3.2 – MCV 94 – MCH 34 – MCHC 36 – Retics 6.8% Labs- cont. • LFTs normal except for indirect bilirubin of 2.2mg/dl • LDH elevated to 290 • Direct anti-globulin (DAT) test is negative Peripheral Blood Smear Peripheral Blood Smear (cont’d) Peripheral Blood Smear (cont’d) Hereditary spherocytosis (HS) • Initially described in 1871 • Increased in persons of northern European descent • In the US: 1 in 5000 • Most cases inherited in an autosomal dominant fashion Structure • The RBC membrane is composed of a lipid bilayer with associated proteins • This is linked to a protein network “membrane cytoskeleton” • This specific structural configuration gives the red blood cell flexibility (to squeeze through capillaries) Membrane Protein Defects- resulting in spherocytes • Spectrin deficiency alone • Spectrin and ankyrin deficiency • Band 3 deficiency • Protein 4.2 defects Pathophysiology • Spherocytes -

Congenital Heinz-Body Haemolytic Anaemia Due to Haemoglobin Hammersmith
Postgrad Med J: first published as 10.1136/pgmj.45.527.629 on 1 September 1969. Downloaded from Case reports 629 TABLE 1 After transfusion Before transfusion 24 hr 72 hr 6 days Haemoglobin (g) ND 7-5 7-6 7-6 PCV 20 29 23 29 Plasma free Hb (mg/100 ml) 241 141 5 6 Bleeding time 14 6 4 1 Clotting time No clot 8 5-5 2 Haemolysins 3±+ - - Serum bilirubin (mg/100 ml) 4-0 4-8 1-3 0-6 Urine volume/24 hr (ml) 970 1750 1500 1300 Haemoglobinuria 4+ 1 + - - Urobilin 2+ 3+ 3 + 1+ Blood urea (mg/100 ml) 30 148 75 32 Serum Na (mEq/l) 128 132 136 140 Serum K (mEq/l) 3-8 4-2 4-6 4-6 Serum HCO3 (mEq/l) 21-2 27-6 28-4 30 0 SGOT Frankel Units ND 110 84 36 SGPT Frankel Units 94 96 68 Acknowledgment Reference We are greatly indebted to Dr P. E. Gunawardena, REID, H.A. (1968) Snake bite in the tropics. Brit. med. J. 3, Superintendent of the National Blood Transfusion Service 359. for his help in this case. Protected by copyright. Congenital Heinz-body haemolytic anaemia due to Haemoglobin Hammersmith N. K. SHINTON D. C. THURSBY-PELHAM M.D., M.R.C.P., M.C.Path. M.D., M.R.C.P., D.C.H. H. PARRY WILLIAMS M.R.C.S., F.R.C.P. Coventry and Warwickshire Hospital and City General Hospital, Stoke-on-Trent http://pmj.bmj.com/ THE ASSOCIATION of haemolytic anaemia with red shown by Zinkham & Lenhard (1959) to be associ- cell inclusion bodies was well recognized at the end ated with an hereditary deficiency of the red cell ofthe Nineteenth Century in workers exposed to coal enzyme glucose-6-phosphate dehydrogenase. -
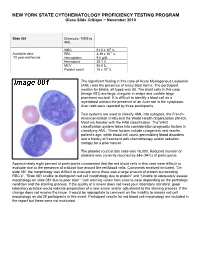
10 11 Cyto Slides 81-85
NEW YORK STATE CYTOHEMATOLOGY PROFICIENCY TESTING PROGRAM Glass Slide Critique ~ November 2010 Slide 081 Diagnosis: MDS to AML 9 WBC 51.0 x 10 /L 12 Available data: RBC 3.39 x 10 /L 72 year-old female Hemoglobin 9.6 g/dL Hematocrit 29.1 % MCV 86.0 fL Platelet count 16 x 109 /L The significant finding in this case of Acute Myelogenous Leukemia (AML) was the presence of many blast forms. The participant median for blasts, all types was 88. The blast cells in this case (Image 081) are large, irregular in shape and contain large prominent nucleoli. It is difficult to identify a blast cell as a myeloblast without the presence of an Auer rod in the cytoplasm. Auer rods were reported by three participants. Two systems are used to classify AML into subtypes, the French- American-British (FAB) and the World Health Organization (WHO). Most are familiar with the FAB classification. The WHO classification system takes into consideration prognostic factors in classifying AML. These factors include cytogenetic test results, patient’s age, white blood cell count, pre-existing blood disorders and a history of treatment with chemotherapy and/or radiation therapy for a prior cancer. The platelet count in this case was 16,000. Reduced number of platelets was correctly reported by 346 (94%) of participants. Approximately eight percent of participants commented that the red blood cells in this case were difficult to evaluate due to the presence of a bluish hue around the red blood cells. Comments received included, “On slide 081 the morphology was difficult to evaluate since there was a large amount of protein surrounding RBC’s”, “Slide 081 unable to distinguish red cell morphology due to protein” and “Unable to adequately assess morphology on slide 081 due to poor stain”. -

TOPIC 5 Lab – B: Diagnostic Tools & Therapies – Blood & Lymphatic
TOPIC 5 Lab – B: Diagnostic Tools & Therapies – Blood & Lymphatic Disorders Refer to chapter 17 and selected online sources. Refer to the front cover of Gould & Dyer for normal blood test values. Complete and internet search for videos from reliable sources on blood donations and blood tests. Topic 5 Lab - A: Blood and Lymphatic Disorders You’ll need to refer to an anatomy & physiology textbook or lab manual to complete many of these objectives. Blood Lab Materials Prepared slides of normal blood Prepared slides of specific blood pathologies Models of formed elements Plaque models of formed elements Blood typing model kits Blood Lab Objectives – by the end of this lab, students should be able to: 1. Describe the physical characteristics of blood. 2. Differentiate between the plasma and serum. 3. Identify the formed elements on prepared slides, diagrams and models and state their main functions. You may wish to draw what you see in the space provided. Formed Element Description / Function Drawing Erythrocyte Neutrophil s e t y c Eosinophils o l u n a r Basophils Leukocytes G e Monocytes t y c o l u n Lymphocytes a r g A Thrombocytes 4. Define differential white blood cell count. State the major function and expected range (percentage) of each type of white blood cell in normal blood. WBC Type Function Expected % Neutrophils Eosinophils Basophils Monocytes Lymphocytes 5. Calculation of the differential count? 6. Define and use in proper context: 1. achlorhydria 5. amyloidosis 2. acute leukemia 6. anemia 3. agnogenic myeloid metaplasia 7. autosplenectomy 4. aleukemic leukemia 8. basophilic stippling 9.