Ganado Site Visit: Mexican Rice Borer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Integrated Pest Management of the Mexican Rice Borer in Louisiana and Texas Sugarcane and Rice Francis P
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2005 Integrated pest management of the Mexican rice borer in Louisiana and Texas sugarcane and rice Francis P. F. Reay-Jones Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Entomology Commons Recommended Citation Reay-Jones, Francis P. F., "Integrated pest management of the Mexican rice borer in Louisiana and Texas sugarcane and rice" (2005). LSU Doctoral Dissertations. 761. https://digitalcommons.lsu.edu/gradschool_dissertations/761 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. INTEGRATED PEST MANAGEMENT OF THE MEXICAN RICE BORER IN LOUISIANA AND TEXAS SUGARCANE AND RICE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the formal requirements for the degree of Doctor of Philosophy in The Department of Entomology by Francis P. F. Reay-Jones B.S., Université Bordeaux 1, 1999 M.S., Université d’Angers/Institut National d’Horticulture, 2001 August 2005 ACKNOWLEDGEMENTS I wish to express my gratitude to my major advisor, Dr. Thomas E. Reagan, for his guidance, motivation, and also for introducing me with great enthusiasm to LSU football and Cajun cuisine. I would like to express my appreciation to my graduate committee, Drs. Benjamin L Legendre, E. -

PERFORMANCE REPORT State: Louisiana Project Number: W-55-25 Project Title: Upland Survey Period Covered: July 1, 2010
PERFORMANCE REPORT State: Louisiana Project Number: W-55-25 Project Title: Upland Survey Period Covered: July 1, 2010 - June 30, 2011 Study Number and Title: V-4, Bobwhite Fall Whistling Surveys Study Objective: To determine relative size and distribution of bobwhite populations in Louisiana. Study Abstract Bobwhite fall whistling counts were conducted statewide along 32 routes (7 additional routes were assumed zeros and 4 others were unable to be run). Fall whistling counts did not differ (P = 0.3482) among the 5 habitat types for 2010. They did, however, differ between 2009 and 2010, with more whistling counts detected in 2009 than in 2010 for both the Northwest mixed shortleaf and loblolly pine-hardwood and Florida Parishes loblolly pine habitats. No coveys were recorded on the Northwest mixed shortleaf and loblolly pine- hardwood or Mississippi/Atchafalaya agricultural belt (0.00 whistles/stop) in 2010 (Tables 1 and 2). Jackson- Bienville WMA, Camp Beauregard, and Vernon District No. 1 routes yielded 0.25, 0.00, and 0.10 whistles/stop, respectively (Table 3). Five (5) summer bobwhite counts were conducted on the Sandy Hollow Wildlife Management Area. Counts ranged from 12-45 (Figure 1). The 2011 mean count was 27.6, which is the same as in 2010. The peak count (45) was lower than the long-term (1986-2010) average peak count of 89.3, but was higher than 8 of the past 10 years (Figure 2). A. Activity: Fall whistling counts were conducted along 37 pre-selected routes to determine relative size and distribution of fall bobwhite populations. The 5 habitat types surveyed were historic longleaf pine, mixed shortleaf and loblolly pine-hardwood, loblolly pine (Florida Parishes), the rice belt and the Mississippi/Atchafalaya agricultural belt. -

A. FINAL Rice Report Cover 9-28-00 #2
Report by the INSTITUTE FOR SCIENCE, TECHNOLOGY AND PUBLIC POLICY George Bush School of Government and Public Service ECOLOGICAL, ECONOMIC AND POLICY ALTERNATIVES FOR TEXAS RICE AGRICULTURE September 25, 2000 Funded by TEXAS WATER RESOURCES INSTITUTE TR-181 Alston, L.T., Lacher, T.E., Slack, R.D., Vedlitz, A., Woodward, R.T., Franklin, J.C., Canzoneri, N., Torres Conkey, A.A., Cowman, D.F., Harris, J., Henry, A., Kennedy, E., Krohn, M.R., Mizell, K., Nicholson, J., Tierce, K., and Wui, Y. (2000). Ecological, economic, and policy alternatives for Texas rice agriculture. A report by the Institute for Science, Technology and Public Policy in the George Bush School of Government and Public Service to the Texas Water Resources Institute/Agricultural Program, Texas A&M University System. (TWRI Report TR-181). This work may not be reproduced in its entirety, nor in part, without the knowledge and permission of its authors. INSTITUTE FOR SCIENCE, TECHNOLOGY AND PUBLIC POLICY A nonpartisan, interdisciplinary public policy research institute. FACULTY RESEARCHERS Letitia T. Alston, Ph.D. • Institute for Science, Technology and Public Policy Thomas E. Lacher, Ph.D. • Wildlife and Fisheries Sciences R. Douglas Slack, Ph.D. • Wildlife and Fisheries Sciences Arnold Vedlitz, Ph.D. • Political Science • Institute for Science, Technology and Public Policy Richard T. Woodward, Ph.D. • Agricultural Economics POST-DOCTORAL RESEARCH ASSOCIATE James C. Franklin, Ph.D. • Political Science • Institute for Science, Technology and Public Policy RESEARCH ASSISTANTS Nicole Canzoneri • Political Science April Ann Torres Conkey • Wildlife and Fisheries Sciences Deborah F. Cowman • Wildlife and Fisheries Sciences Jeanine Harris • Political Science April Henry • Wildlife and Fisheries Sciences Elizabeth Kennedy • Forestry Sciences Michelle R. -

Warehouses Licensed Under the U.S. Warehouse
Warehouses United States Department of Agriculture Licensed Under the Farm Service U.S. Warehouse Act Agency Deputy Administrator for Commodity As of December 31, 2014 Operations LICENSED AND BONDED WAREHOUSE The Storage of Agricultural Products Is Locked Into the Marketing System Through -- Farmers Who Produce Warehouse Operators Who Store Bankers Who Loan Merchants Who Buy and Sell Processors Who Manufacture Consumers Who Use Users of Electronic Warehouse Receipts and other Electronic Documents With Assurance of Return of the Stored Commodity in Marketable Condition. And the Key Is Integrity of the Warehouse Operator and the Warehouse Receipt Through -- Financial Responsibility Warehouse Facilities and Practices Management’s Ethics and Capabilities Licensing Bonding Examination Electronic Providers March 2015 BACKGROUND INFORMATION LEGISLATIVE The United States Warehouse Act (USWA) or the Act was originally passed by AUTHORITY the Congress on August 11, 1916. On November 9, 2000, the President signed the Grain Standards and Warehouse Improvement Act of 2000 (Public Law 106-472) which amended the USWA in its entirety and modernized the regulation of Federal warehouses to better reflect current trade and operational practices. The 2000 Act authorizes the Secretary of Agriculture to license and examine public warehouse operators that store agricultural products. It also gives the Secretary broad authority to improve the administration of various aspects involving the trade of agricultural products under the purview of the USWA, including the use, establishment and maintenance of electronic warehouse receipts and other electronic documents for all agricultural products. The USWA is considered a “permissive regulatory act.” It is permissive because it only applies to warehouse operators who voluntarily apply for licensing and regulatory because each licensee must operate under its provisions and be subject to the regulations and licensing agreements administered by the USWA. -

B.R. Wells Rice Research Studies 2012
B.R. Wells Rice Research Studies 2012 R.J. Norman and K.A.K. Moldenhauer, editors A R K A N S A S A G R I C U L T U R A L E X P E R I M E N T S T A T I O N August 2013 Research Series 609 This publication is available on the Internet at http://arkansasagnews.uark.edu/1356.htm Cover Photo: Rice test plots at the Rice Research and Extension Center facilities, Stuttgart, Ark. Photo credit: University of Arkansas System Division of Agriculture. Layout and editing by Marci A. Milus Technical editing and cover design by Gail Halleck Arkansas Agricultural Experiment Station, University of Arkansas System Division of Agriculture, Fayetteville. Mark J. Cochran, Vice President for Agriculture. Clarence E. Watson, Associate Vice-President for Agriculture–Research and Director, AAES. MG400CS5/CS6. The University of Arkansas System Division of Agriculture follows a nondiscriminatory policy in programs and employment. ISSN:1941-2177 CODEN:AKAMA6 B.R. Wells RICE Research Studies 2 0 1 2 R.J. Norman and K.A.K. Moldenhauer, editors University of Arkansas System Division of Agriculture Arkansas Agricultural Experiment Station Fayetteville, Arkansas 72701 DEDICATED IN MEMORY OF Bobby R. Wells Bobby R. Wells was born July 30, 1934, at Wickliffe, Ky. He received his B.S. degree in agriculture from Murray State University in 1959, his M.S. degree in agronomy from the University of Arkansas in 1961, and his Ph.D. in soils from the University of Missouri in 1964. Wells joined the faculty of the University of Arkansas in 1966 after two years as an assistant professor at Murray State University. -

2015 Winter Whole Grain
VOL 2. NO 1 AND 2 I WINTER 2015 2015 USA RICE OUTLOOK CONFERENCE: WISH YOU WERE HERE! also in this issue: 2015 USA RICE OUTLOOK CONFERENCE INTERNATIONAL DECEMBER 9-11 TRADE: SHERATON NEW ORLEANS TPP – Why Should Rice Care? NEW ORLEANS, LA PAGE 6 CONFERENCE BROCHURE SPECIAL SECTION REP. RALPH ABRAHAM (R-LA) PAGE 8 INTERNATIONAL PROMOTION: Fortified Rice Good for Food Aid PAGE 22 USA Rice Financial Report PAGE 28 FROM THE CHAIRMAN’S FARM: VOL 2. NO 1 & 2 I WINTER 2015 December is a Time to Meet and Help BY DOW BRANTLEY who we are: IT’S DECEMBER WHICH MEANS IT’S TIME FOR THE USA EDITOR: RICE OUTLOOK CONFERENCE — an exciting and valuable annual Michael Klein gathering of the rice industry. We come together, this year in New [email protected] Orleans, to hear interesting speakers talk not just about the outlook for rice in our states and around the world, but to exchange ideas, concepts, DEPUTY EDITOR: and information, and to help each other achieve our common goal: Deborah Willenborg being a part of a profitable and sustainable rice industry. [email protected] Speaking of helping people, I’ve just returned from a trip to Havana, Cuba, where USA Rice participated in the Havana Trade CONTRIBUTORS: Show, and if there was ever a group of people that need our help, it’s Rebecca Bratter, Bob Cummings, Donna Dittmann, Jim Guinn, Colleen the Cuban people. Klemczewski, Meghan Mahoney, Don I know there are people right here at home that need our help too, Molino, Sarah Moran, Linda Sieh and I personally do what I can, and I know you do too. -
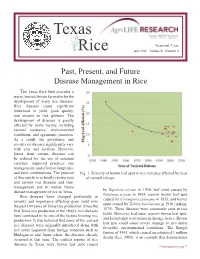
Texas Rice June 2010 Volume X Number 4
Texas Beaumont, Texas Rice June 2010 Volume X Number 4 Past, Present, and Future Disease Management in Rice The Texas Rice Belt provides a warm, humid climate favorable for the development of many rice diseases. Rice diseases cause significant reductions in yield, grain quality, and income to rice growers. The development of diseases is greatly affected by many factors including varietal resistance, environmental conditions, and agronomic practices. As a result, the prevalence and severity of diseases significantly vary with year and location. However, losses from certain diseases can be reduced by the use of resistant varieties, improved practices and management, and effective fungicides and their combinations. The purpose Fig. 1. Severity of brown leaf spot in rice varieties affected by year of this article is to briefly review past of varietal release. and current rice diseases and their management and to outline future by Bipolaris oryzae in 1934, leaf smut caused by disease management of rice in Texas. Entyloma oryzae in 1934, narrow brown leaf spot Rice diseases have changed profoundly in caused by Cercospora janseana in 1935, and kernel severity and importance affecting grain yield over smut caused by Tilletia barclayana in 1936 (Atkins, the past 100 years of Texas rice production. Since the 1975). These diseases were commonly seen in rice first Texas rice production in the 1800’s, rice diseases fields. However, leaf smut, narrow brown leaf spot, have continued to be one of the factors limiting rice and kernel smut were minor in damage to rice. Brown production. It was believed that some of the earliest leaf spot could cause severe damage to rice under rice diseases were originally introduced along with favorable environmental conditions. -

Chapter 7 SPACE, TIME, and VARIATION S
Chapter 7 SPACE, TIME, AND VARIATION s G gi l~ oj . some species can Indeed be said to dl C( have had a single and sudden origin, local o Ized and capable of being located. With is others, however, the or/gin Is no origin at 01/ d, but a gradual transformation extending over wide areas and long periods and shifting Its focus In the course of time. Between the two Is every gradation. D.C. Darlington and EK Janakl Amma!, 1945 1 n s' b c s s e ( '\;, Space, Time, and Variation! KINDS OF PATTERNS OF VARIAnON Geographic patterns of variation have historically been used to trace the ori gin and evolution of cultivated plants. We have seen that Vavilov (1926. 1949/50) thought that areas of maximum genetic diversity represented centers of origin and that the origin of a crop could be identified by the simple proce dure of analyzing variation patterns and plotting regions where diversity was concentrated. It turned out that centers of diversity are not the same as centers of origin. yet many crops do exhibit centers of diversity. The phenomenon is real and requires explanation. What causes variation to accumulate in secon dary centers is not too well understood, but some observable factors are: 1. A long history of continuous cultivation. 2. Ecological diversity. many habitats accommodate many races. 3. Human diversity, different tribes are attracted to different races of the crop. 4. Introgression with wild and weedy relatives or between different races of a crop. There may be other causes. but the reasons for secondary centers are hu man. -

April to June, 1917, Inclusive : Index to Volume
TWO SECTIONS--SECTION TWO (Issue of July 14 1917) The Randal attat r nide INCLUDING Bank St Quotation Section Railway & Industrial Section Electric Railway Section Railway Earnings Section Bankers' Convention Section State and City Section A WEEKLY NEWSPAPER Representing the Industrial Interests of the United States. APRIL TO JUNE, 1917, INCLUSIVE. VOLUME 104—PART 2 WILLIAM B. DANA COMPANY, PUBLISHERS FRONT, PINE & DEPEYSTER STS., NEW YORK. Digitized for FRASER http://fraser.stlouisfed.org/ Federal Reserve Bank of St. Louis Copyrighted in 1917, according to Act of Congress, by WILLIAM B. DANA COMPANY in office of Librarian of Congress, Washington, D. C. Digitized for FRASER http://fraser.stlouisfed.org/ Federal Reserve Bank of St. Louis APRIL-JUNE) 1917.] INDEX. III INDEX TO VOLUME•104 PART 2. APRIL 1 TO JUNE 30 1917. EDITORIAL AND COMMUNICATED ARTICLES. Page. Page. Page A cceptances-See Federal Reserve Matters. pabson Warns of Socialistic Changes After British-(Coucluded) ,racworth, W. M., Before Newlands Corn- 2-• War 2595 Food Controller Ownership Warns of Coming Priva- mittee on Government of Baker, Raymond T., Confirmed as Director tion-Increase of Acreage 1670 Roads 2079 of Mint 1343 Labor Commission, Capital and Labor Agricultural Development, N. Y. Bankers' Baltimore & Ohio RR., Commerce Commis- Commune With 1964 Association on 1448 sion Ruling on Embargo, Practice of 2610 Labor Commission Preparedness Measures, Farm- Co-operating With Agricultural Bank Clearings in Canada-See Canada American Labor 2418 ers Urged to Join in 1340 Bank Clearings at Leading Cities 1361, Labor Strike Among Agricultural Resources, N. Y. State Bank- Munition Workers Co-operate 1772, 2316 and London Bus Drivers 1942 ing Institutions Urged to Bank Clearings by Telegraph_1361, 1772, 2316 Lens (French Coal City) Surrounded__ _ _2582 with Farmers in Developing 1755 Bank Clearings in U. -

The American Legion Monthly Is the Official Publication of the American Legion and the American Legion Auxiliary and Is Owned Exclusively
QlfieMERI25 Cents CAN EGION OHonthlj/ Meredith Nicholson - Lorado Taft Arthur Somers Roche — Who wants to live on a poorly lighted street? Nobody who knows the advantages of modern lighting—the safety for drivers and pedestrians the protection against crime—the evidence of a desirable residential area. The service of General Electric's street-lighting To-day, no street need be dark, for good specialists are always at the command of communities street lighting costs as little as two dollars a year interested in better light- per capita; and for that two dollars there is a ing. In cooperation with your local power company, substantial increment in property value they will suggest appropri- ate installations, and give you the benefit of their It isn't a question whether you can afford long experience in the de- sign and operation of street- good street lighting, but— can you afford not lighting and electric traffic- control systems. to have it? GENERAL ELECTRIC S. F. ROTHAFEL— the famous Roxy—of Roxy and His Gang — builder of the world's finest theatre—known and loved by hundreds of thousands—not only for his splendid enter- tainments but for his work in bringing joy and sunshine into the lives of so many in hos- pitals and institutions. Never too busy to help—Roxy keeps himself in good physical con- dition by proper rest—on a Simmons Beautyrest Mattress and Ace Spring Anyone who has to take his rest in concentrated doses is mighty particular " about how he beds himself down doesn't know "Roxy"— his says springy wire coils and its soft mattress * ^ voice on the air has cheered millions layers is the result. -

A Training Guide for in Situ Conservation On-Farm
A Training Guide for In Situ Conservation On-farm Version 1 D.I Jarvis, L. Myer, H. Klemick, L. Guarino, M. Smale, A.H.D. Brown, M. Sadiki, B. Sthapit and T. Hodgkin SWISS AGENCY FOR DEVELOPMENT AND COOPERATION SDC IDRC Netherlands Ministry of Foreign Affairs Development Cooperation Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ) GmbH Bundesministerium für Wirtschaftliche Zusammenarbeit, Germany IPGRI is a Centre A Training Guide for In Situ Conservation On-farm Version 1 D.I Jarvis, L. Myer, H. Klemick, L. Guarino, M. Smale, A.H.D. Brown, M. Sadiki, B. Sthapit and T. Hodgkin With the collaboration of : S. Achtar P. Eyzaguirre T. Quinones-Vega A. Amri D. Fanissi K. R’hrib L. Arias-Reyes M. Fernandez R. Rana Z. Asfaw E. Friis-Hansen V. Ramanatha Rao G. Ayad D. Gauchan D. Rijal J. Bajracharya N.P. Ha K. Riley R. Balaghi N.N. Hue J. Rodriguez D. Balma M. Ibnou-Ali R. Salazar B. Baniya K. Joshi E. Sauri-Duch M.O. Belem O. Kabore M. Sawasogo A. Birouk S. Khatiwada R. Sevilla-Panizo A. Bouizgaren A. King K.K. Sherchand P. Bramel-Cox D. Lope-Alzina A. Subedi S. Brush M. Mahdi M. Taghouti L. Burgos-May I. Mar A. Tan J. Canul-Ku F. Marquez-Sanchez A. Teshome F. Castillo-Gonzalez P.N. Mathur I. Thorman S. Ceccarelli H. Mellas H.Q. Tin P. Chaudhary N.K. Motiramani P. Tiwari J.-L. Chavez-Servia C. Morales-Valderrama L.N. Trinh V. Cob-Vicab A. Mudwari R. Tripp P.H. Cuong F. Nassif M. Upadhyay N.N. -

Oryza Glaberrima
African rice (Oryza glaberrima) cultivation in the Togo Hills: ecological and socio-cultural cues in farmer seed selection and development and socio-cultural cues in farmer seed selection development African rice ( Oryza glaberrima ) cultivation in the Togo Hills: ecological Togo ) cultivation in the Béla Teeken Béla Béla Teeken African rice (Oryza glaberrima) cultivation in the Togo Hills: ecological and socio-cultural cues in farmer seed selection and development Béla Teeken Thesis committee Promotors Prof. Dr P. Richards Emeritus professor of Technology and Agrarian Development Wageningen University Prof. Dr P.C. Struik Professor of Crop Physiology Wageningen University Co-promotors Dr H. Maat Assistant Professor Knowledge, Technology and Innovation group Wageningen University Dr E. Nuijten Senior Researcher Plant Breeding & Sustainable Production Chains Louis Bolk Institute Other members Prof. Dr H.A.J. Bras, Wageningen University Prof. Dr S. Hagberg, Professor of Cultural Anthropology, Uppsala University, Sweden Dr T.J.L. van Hintum, Wageningen University Dr S. Zanen, Senior Trainer Consultant, MDF Training & Consultancy, Ede This research was conducted under the auspices of the Wageningen School of Social Sciences (WASS). African rice (Oryza glaberrima) cultivation in the Togo Hills: ecological and socio-cultural cues in farmer seed selection and development Be´la Teeken PHD Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr A.P.J. Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Tuesday 1 September 2015 at 4 p.m. in the Aula. Béla Teeken African rice (Oryza glaberrima) cultivation in the Togo Hills: ecological and socio-cultural cues in farmer seed selection and development 306 pages PhD thesis, Wageningen University, Wageningen, NL (2015) With references, with summaries in English and Dutch ISBN: 978-94-6257-435-9 Abstract Teeken B (2015).