Moringua Edwardsi (Moringuidae: Anguilliformes): Cranial Specialization for Head-First Burrowing?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Weekly Report, Leg 1 (01.04
Weekly Report, Leg 1 (01.04. to 05.04.2015) Research Vessel Maria S. Merian left Pennos Wharf in St. George’s, Bermuda as scheduled on 01.04.2015 at 9:00 o´clock after four hours of fuel bunkering in the dockyards. With a strong breeze she steamed from Bermuda towards the first sampling station on our westernmost transect at 70°W, 30°N. The first trial run of all sampling gear was successful except for the CTD probe (Figure 1), which due to some defect showed strong deviations from the expected temperature and salinity data. After intensive efforts and various steps to repair the probe, the problem could be solved and all data were successfully recalibrated. The deployment of the multinet, the two Isaaks-Kidd midwater trawls (0.5 and 5 mm mesh size) (Figure 2), the 1 m2 MOCNESS (Figure 3) and Manta trawls worked fine, although is was decided to run the IKMTs from the stern instead of the starboard side. The cooperation with the ship´s crew is excellent. Station planning follows the programme suggested in the ship´s research proposal and is carried out in close communication with the captain and scientists. Each station of the first transect includes the deployment of a CTD and an IKMT. In addition, MOCNESS and multinet, respectively, as well as the 5-mm IKMT are used alternatively at two neighbouring stations. The Manta trawl may be deployed parallel to other trawled gear, captain permitting. The tedious sorting of the plankton samples of all gears is carried out directly after each catch. -

Anguilliformes and Saccopharyngiformes
Anguilliformes and Saccopharyngiformes Selected meristic characters in species belonging to the orders Anguilliformes or Saccopharyngiformes whose adults or larvae have been collected in the study area. Classification sequence follows Böhlke, 1989. Characters pertain to leptocephali, unless otherwise indicated. Sources: Smith, 1989a; 1989b (and all chapters therein); vert = vertebrae. Last Vertical No. of Gut Family Total Preanal Predorsal Blood Vessel Loops or Species Myomers Myomeres Myomers @ Myomere # Swellings Anguilliformes – Anguillidae Anguilla anguilla 111–119 – – – 0 Anguilla rostrata 103–111 68–73 61–66 44–47 0 Moringuidae Neoconger mucronatus 93–109 49–61 39–56 50–59 1 Moringua edwardsi 109–123 72–82 79–87 70–79 1 Muraenidae Anarchias similis 105–114 52–59 96–104 53–57 0 Gymnothorax funebris (adult) 137–142 – – – – Gymnothorax miliaris 120–125 69–74 68–73 64–69 0 Gymnothorax moringa 137–143 66–74 52–61 60–72 0 Gymnothorax ocellatus 136–150 85–101 22–32 77–87 0 Gymnothorax vicinus 131–142 60–68 53–63 60–67 0 Monopenchelys acuta 128–134 54–57 78–82 60–62 0 Uropterygius macularius 118–123 71–77 107–114 65–67 0 Synaphobranchidae Dysomma anguillare 118–128 57–62 45–48 60–64 6 Ilyophis brunneus (adult) 145–151 vert – – – – Leptocephalus dolichorhynchus 128–136 61–71 – – 1 Leptocephalus proboscoideus 128–134 72–79 69 59–62 0 Simenchelys parasiticus (adult) 115–117 vert – – – – Synaphobranchus affinis 128–139 vert – – – – Synaphobranchus bathybius (adult) 126–140 vert – – – – Synaphobranchus capensis (adult) 164–173 vert – – – – Synaphobranchus kaupi 143–154 98–107 (see species) 68–73 0 Synaphobranchus sp. -

<I>Moringua Edwardsi</I>
BULLETIN OF MARINE SCIENCE, 29(1): 1-18, 1979 EARLY LIFE-HISTORY OF THE EEL MORINGUA EDWARDSI (PISCES, MORINGUIDAE) IN THE WESTERN NORTH ATLANTIC P. H. J. Castle ABSTRACT The eel Morillgua edwardsi (Jordan and Bollman, 1889) is known principally from immature specimens in the western North Atlantic from Bermuda southwards to Atlantic Panama and Colombia. Its distinctive larva, earlier recognized as Leptocephalus diptychus Eigenmann, 1900, has about seven alternating, midlateral melanophores and also one in front of the anus. Larvae have 110-124 myomeres, hatch at about 5 mm TL and reach full growth at 50 mm TL, a process which takes 3-5 months, before metamorphosis begins. They live in the upper 35 ill and occur over a broad area of the western North Atlantic encompassing 1O°-40oN and 40o_88°W but those of about 10 mm TL occur only near the Caribbean Islands. Spawning is suggested to occur in the Caribbean the year round but principally at monthly intervals from November to April. Some larvae are dispersed out into the Atlantic by the Gulf Stream. This pattern of distribution and dispersal is similar to that of the only other Atlantic moringuid Neoconger mucronatus Girard, 1859. At a time when the classification of the 1968) to clarify the nomenclature of the eels is undergoing critical scrutiny, major Moringuidae pointed out that at least one difficulties are still being presented by the character (number of vertebrae) would family Moringuidae. Moringuid eels are need to be considered in any re-appraisal readily captured with piscicides in many of moringuid classification. -
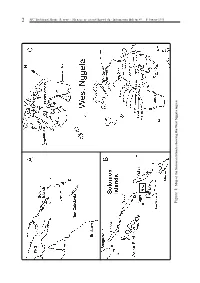
An Analysis of the West Nggela (Solomon Islands) Fish Taxonomy
2 SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 Map of the Solomon Islands showing West Nggela region Figure 1: Figure SPC Traditional Marine Resource Management and Knowledge Information Bulletin #9 – February 1998 3 What’s in a name? An analysis of the West Nggela (Solomon Islands) fish taxonomy. by Simon Foale 1 Introduction Lobotidae, Gerreidae, Sparidae, Ephippidae, Chaetodontidae, Pomacentridae, Cirhitidae, Accurate knowledge about the behaviour, biol- Polynemidae, Labridae, Opistognathidae, ogy and ecology of organisms comprising marine Trichonotidae, Pinguipedidae, Blenniidae, fisheries is a vital prerequisite for their manage- Gobiidae, Microdesmidae, Zanclidae, Bothidae, ment. Before beginning any study on local knowl- Pleuronectidae, and Soleidae. edge of marine fauna, a working knowledge of The English names of many species of fish vary their local names must be obtained. Moreover, a quite a bit, even within one country such as great deal of local knowledge can often emerge in Australia. For most of the species listed in the very process of obtaining names (Ruddle, Appendix 1, I have used the English names given 1994). A detailed treatment of the local naming by Randall et al. (1990). For species not included in system of West Nggela marine fauna is given in Randall et al. (1990), names from Kailola (1987a, b, this paper. 1991) were used. Methods Results Local names of fish were collected by asking Appendix 1 contains 350 unique Nggela folk people to provide the Nggela names for fishes taxa for cartilaginous and bony fishes, together from photographs in books featuring most of the with the scientific (Linnean) taxa they correspond common Indo-Pacific species (Randall et al., 1990 to and, where available, a brief note describing an and Myers, 1991). -

Mariana-FEP-SAFE-Rep
ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT: MARIANA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2016 Western Pacific Regional Fishery Management Council 1164 Bishop St., Suite 1400 Honolulu, HI 96813 PHONE: (808) 522-8220 FAX: (808) 522-8226 www.wpcouncil.org The ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT for the MARIANA ARCHIPELAGO FISHERY ECOSYSTEM 2016 was drafted by the Fishery Ecosystem Plan Team. This is a collaborative effort primarily between the Western Pacific Regional Fishery Management Council, NMFS-Pacific Island Fisheries Science Center, Pacific Islands Regional Office, Division of Aquatic Resources (HI) Department of Marine and Wildlife Resources (AS), Division of Aquatic and Wildlife Resources (Guam), and Division of Fish and Wildlife (CNMI). This report attempts to summarize annual fishery performance looking at trends in catch, effort and catch rates as well as provide a source document describing various projects and activities being undertaken on a local and federal level. The report also describes several ecosystem considerations including fish biomass estimates, biological indicators, protected species, habitat, climate change and human dimensions. Information like marine spatial planning and best scientific information available for each fishery are described. This report provides a summary of annual catches relative to the Annual Catch Limits established by the Council in collaboration with the local fishery management agencies. Edited By: Marlowe Sabater, Asuka Ishizaki, Rebecca Walker, and Sylvia Spalding, WPRFMC This document can cited as follows: WPRFMC 2017. Annual Stock Assessment and Fishery Evaluation Report for the Mariana Archipelago Fishery Ecosystem Plan 2016. Sabater, M., Ishizaki, A., Walker, R., Spalding, S. (Eds.) Western Pacific Regional Fishery Management Council. -

Deep-Ocean Origin of the Freshwater Eels
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Biol. Lett. (2010) 6, 363–366 2003) occupy more basal positions in the published doi:10.1098/rsbl.2009.0989 phylogenies, Tsukamoto et al. (2002) hypothesized Published online 6 January 2010 two phases in the evolution of catadromous migrations Evolutionary biology of freshwater eels: (i) their migratory behaviour originated in tropical ocean areas and (ii) that tropical species subsequently expanded their ‘migration loops’ Deep-ocean origin of the (representative migratory pathway of a species), and began to use fresh waters at higher latitudes for their freshwater eels growth (figure 1a). This resulted in the diversification of freshwater eels to include temperate species that Jun G. Inoue1,*,†, Masaki Miya2,*, Michael make long migrations back to their tropical spawning J. Miller1, Tetsuya Sado2, Reinhold Hanel3, areas. This argument is consistent with the Gross Kiyotaka Hatooka4, Jun Aoyama1, Yuki Minegishi1, 1 1 et al. (1988) prediction that catadromy has evolved in Mutsumi Nishida and Katsumi Tsukamoto low-latitude tropical areas where productivity in fresh- 1Ocean Research Institute, The University of Tokyo, water exceeds that in the ocean. In contrast to eels, the Tokyo 164-8639, Japan 2Natural History Museum and Institute, Chiba, Chiba 266-8682, Japan freshwater origin of anadromous salmon has been 3Johann Heinrich von Thu¨nen-Institut 22767, Hamburg, Germany demonstrated (Ishiguro et al. 2003), which indicates 4Osaka Museum of Natural History, Osaka 546-0034, Japan that they expanded their life histories to include the *Authors for correspondence ( [email protected], [email protected]) use of the ocean for growth while still returning to †Present address: University College London, London WC1E 6BT, UK. -

An Annotated Checklist of Eels in Bago River, Negros Occidental, Philippines
AsianVol.Vol. 11 Journal No.No. 11 DecemberDecember of Biodiversity 20102010 ISSN: 2094-15019 Asian Journal of Biodiversity CHED Accredited Research Journal, Category A Art. #105, pp. 126-138 Print ISSN 2094-1519 • Electronic ISSN 2244-0461 doi: http://dx.doi.org/10.7828/ajob.v1i1.105 An Annotated Checklist of Eels in Bago River, Negros Occidental, Philippines ABNER A. BUCOL [email protected] Silliman University Angelo King Center for Research and Environmental Management, Dumaguete City CARMEN C. MENES [email protected] JOJI D. LINAUGO [email protected] La Consolacion College-Bacolod, Bacolod City Date Submitted: October 19, 2010 Plagiarism Detection: Passed Final Revision Complied: Nov. 7, 2010 Original: 91.30% Gunning Fog Index: 11.59 English Writing Readability: 44.18 Abstract - The eels occurring in Bago River, Negros Occidental, Philippines are briefly annotated. Order Synbranchiformes is represented by the swamp eel Ophisternon bengalense while Anguilliformes (or true-eels) consist of 10 species belonging to four families. Snake-eels (Ophichthidae) consist of seven species while Freshwater Eels (Anguillidae), Spaghetti Eels (Moringuidae), Moray Eels (Muraenidae) are represented by a single species each. Keywords: Eels, river, brackish water, freshwater, Negros Occidental 126 Checklist of Fishes Found in the Fresh and Brackish Waters... Bucol & Carumbanan INTRODUCTION Eels are a diverse group of fishes characterized by having elongate, snake-like or worm-like bodies (Smith & McCosker 1999) and, except the synbranchids, all have leptocephalic larval stage (Smith 1979; Smith & Castle 1972; Castle 1966, 1968; Miller & Tsukamoto 2004). The eels in the Philippines have been the subject of numerous studies. Among the earliest publications included, but not limited to, the following: Bleeker (1864), Herre (1923, 1924). -

Early Life-History of the Eel <I>Moringua Edwardsi</I> (Pisces, Moringuidae) in the Western North Atlantic
BULLETIN OF MARINE SCIENCE, 29(1): 1-18, 1979 EARLY LIFE-HISTORY OF THE EEL MORINGUA EDWARDSI (PISCES, MORINGUIDAE) IN THE WESTERN NORTH ATLANTIC P. H. J. Castle ABSTRACT The eel Morillgua edwardsi (Jordan and Bollman, 1889) is known principally from immature specimens in the western North Atlantic from Bermuda southwards to Atlantic Panama and Colombia. Its distinctive larva, earlier recognized as Leptocephalus diptychus Eigenmann, 1900, has about seven alternating, midlateral melanophores and also one in front of the anus. Larvae have 110-124 myomeres, hatch at about 5 mm TL and reach full growth at 50 mm TL, a process which takes 3-5 months, before metamorphosis begins. They live in the upper 35 ill and occur over a broad area of the western North Atlantic encompassing 1O°-40oN and 40o_88°W but those of about 10 mm TL occur only near the Caribbean Islands. Spawning is suggested to occur in the Caribbean the year round but principally at monthly intervals from November to April. Some larvae are dispersed out into the Atlantic by the Gulf Stream. This pattern of distribution and dispersal is similar to that of the only other Atlantic moringuid Neoconger mucronatus Girard, 1859. At a time when the classification of the 1968) to clarify the nomenclature of the eels is undergoing critical scrutiny, major Moringuidae pointed out that at least one difficulties are still being presented by the character (number of vertebrae) would family Moringuidae. Moringuid eels are need to be considered in any re-appraisal readily captured with piscicides in many of moringuid classification. -

Mariana Archipelago Fishery Ecosystem Plan 2017
ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT: MARIANA ARCHIPELAGO FISHERY ECOSYSTEM PLAN 2017 Western Pacific Regional Fishery Management Council 1164 Bishop St., Suite 1400 Honolulu, HI 96813 PHONE: (808) 522-8220 FAX: (808) 522-8226 www.wpcouncil.org The ANNUAL STOCK ASSESSMENT AND FISHERY EVALUATION REPORT for the MARIANA ARCHIPELAGO FISHERY ECOSYSTEM 2017 was drafted by the Fishery Ecosystem Plan Team. This is a collaborative effort primarily between the Western Pacific Regional Fishery Management Council, NMFS-Pacific Island Fisheries Science Center, Pacific Islands Regional Office, Division of Aquatic Resources (HI) Department of Marine and Wildlife Resources (AS), Division of Aquatic and Wildlife Resources (Guam), and Division of Fish and Wildlife (CNMI). This report attempts to summarize annual fishery performance looking at trends in catch, effort and catch rates as well as provide a source document describing various projects and activities being undertaken on a local and federal level. The report also describes several ecosystem considerations including fish biomass estimates, biological indicators, protected species, habitat, climate change, and human dimensions. Information like marine spatial planning and best scientific information available for each fishery are described. This report provides a summary of annual catches relative to the Annual Catch Limits established by the Council in collaboration with the local fishery management agencies. Edited By: Marlowe Sabater, Asuka Ishizaki, Thomas Remington, and Sylvia Spalding, WPRFMC. This document can be cited as follows: WPRFMC, 2018. Annual Stock Assessment and Fishery Evaluation Report for the Mariana Archipelago Fishery Ecosystem Plan 2017. Sabater, M., Ishizaki, A., Remington, T., Spalding, S. (Eds.) Western Pacific Regional Fishery Management Council. -

Family-Group Names of Fossil Fishes
© European Journal of Taxonomy; download unter http://www.europeanjournaloftaxonomy.eu; www.zobodat.at European Journal of Taxonomy 466: 1–167 ISSN 2118-9773 https://doi.org/10.5852/ejt.2018.466 www.europeanjournaloftaxonomy.eu 2018 · Van der Laan R. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:1F74D019-D13C-426F-835A-24A9A1126C55 Family-group names of fossil fi shes Richard VAN DER LAAN Grasmeent 80, 1357JJ Almere, The Netherlands. Email: [email protected] urn:lsid:zoobank.org:author:55EA63EE-63FD-49E6-A216-A6D2BEB91B82 Abstract. The family-group names of animals (superfamily, family, subfamily, supertribe, tribe and subtribe) are regulated by the International Code of Zoological Nomenclature. Particularly, the family names are very important, because they are among the most widely used of all technical animal names. A uniform name and spelling are essential for the location of information. To facilitate this, a list of family- group names for fossil fi shes has been compiled. I use the concept ‘Fishes’ in the usual sense, i.e., starting with the Agnatha up to the †Osteolepidiformes. All the family-group names proposed for fossil fi shes found to date are listed, together with their author(s) and year of publication. The main goal of the list is to contribute to the usage of the correct family-group names for fossil fi shes with a uniform spelling and to list the author(s) and date of those names. No valid family-group name description could be located for the following family-group names currently in usage: †Brindabellaspidae, †Diabolepididae, †Dorsetichthyidae, †Erichalcidae, †Holodipteridae, †Kentuckiidae, †Lepidaspididae, †Loganelliidae and †Pituriaspididae. -

Fishes of the World
Fishes of the World Fishes of the World Fifth Edition Joseph S. Nelson Terry C. Grande Mark V. H. Wilson Cover image: Mark V. H. Wilson Cover design: Wiley This book is printed on acid-free paper. Copyright © 2016 by John Wiley & Sons, Inc. All rights reserved. Published by John Wiley & Sons, Inc., Hoboken, New Jersey. Published simultaneously in Canada. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at www.wiley.com/go/permissions. Limit of Liability/Disclaimer of Warranty: While the publisher and author have used their best efforts in preparing this book, they make no representations or warranties with the respect to the accuracy or completeness of the contents of this book and specifically disclaim any implied warranties of merchantability or fitness for a particular purpose. No warranty may be createdor extended by sales representatives or written sales materials. The advice and strategies contained herein may not be suitable for your situation. -

Introduction to the Systematics and Biodiversity of Eels (Orders Anguilliformes and Saccopharyngiformes) of Taiwan
Zootaxa 4060 (1): 005–018 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2015 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.4060.1.3 http://zoobank.org/urn:lsid:zoobank.org:pub:E35316E7-6D4C-458C-8E42-A11596E519DD Introduction to the systematics and biodiversity of eels (orders Anguilliformes and Saccopharyngiformes) of Taiwan HSUAN-CHING HO1,2, JOHN E. MCCOSKER3, DAVID G. SMITH4 & KWANG-TSAO SHAO5* 1National Museum of Marine Biology & Aquarium, Pingtung, Taiwan 2Institute of Marine Biology, National Dong Hwa University, Pingtung, Taiwan 3California Academy of Sciences, San Francisco, California, U.S.A. 4Smithsonian Institution, Museum Support Center, Suitland, MD, U.S.A. 5Biodiversity Research Center, Academia Sinica, Taipei, Taiwan *Corresponding author: E-mail: [email protected] Abstract The eel fauna (orders Anguilliformes and Saccopharyngiformes) of Taiwan is one of the richest in the world. Recent ge- netic and morphological studies have improved the taxonomic resolution and increased the known diversity of the eels of Taiwan, and the overall diversity is comparable to that of adjacent marine zoogeographic regions with rich biodiversity, such as Australia and the Philippines. In this special issue, we verified the historical records and examined numerous re- cently collected specimens, and conclude that the eel fauna of Taiwan is represented by 207 species in 75 genera and 14 families, with several undescribed species still likely to be discovered. The Muraenidae (71 species), Ophichthidae (60), Congridae (29) and Synaphobranchidae (17) are the most abundant and species-rich in Taiwanese waters. We add 42 spe- cies to the Taiwanese fish fauna, including one new genus and 13 new species.