Effect of Mexiletine on Muscle Stiffness In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 2. 2012 AGS Beers Criteria for Potentially
Table 2. 2012 AGS Beers Criteria for Potentially Inappropriate Medication Use in Older Adults Strength of Organ System/ Recommendat Quality of Recomm Therapeutic Category/Drug(s) Rationale ion Evidence endation References Anticholinergics (excludes TCAs) First-generation antihistamines Highly anticholinergic; Avoid Hydroxyzin Strong Agostini 2001 (as single agent or as part of clearance reduced with e and Boustani 2007 combination products) advanced age, and promethazi Guaiana 2010 Brompheniramine tolerance develops ne: high; Han 2001 Carbinoxamine when used as hypnotic; All others: Rudolph 2008 Chlorpheniramine increased risk of moderate Clemastine confusion, dry mouth, Cyproheptadine constipation, and other Dexbrompheniramine anticholinergic Dexchlorpheniramine effects/toxicity. Diphenhydramine (oral) Doxylamine Use of diphenhydramine in Hydroxyzine special situations such Promethazine as acute treatment of Triprolidine severe allergic reaction may be appropriate. Antiparkinson agents Not recommended for Avoid Moderate Strong Rudolph 2008 Benztropine (oral) prevention of Trihexyphenidyl extrapyramidal symptoms with antipsychotics; more effective agents available for treatment of Parkinson disease. Antispasmodics Highly anticholinergic, Avoid Moderate Strong Lechevallier- Belladonna alkaloids uncertain except in Michel 2005 Clidinium-chlordiazepoxide effectiveness. short-term Rudolph 2008 Dicyclomine palliative Hyoscyamine care to Propantheline decrease Scopolamine oral secretions. Antithrombotics Dipyridamole, oral short-acting* May -

Spectrum of CLCN1 Mutations in Patients with Myotonia Congenita in Northern Scandinavia
European Journal of Human Genetics (2001) 9, 903 ± 909 ã 2001 Nature Publishing Group All rights reserved 1018-4813/01 $15.00 www.nature.com/ejhg ARTICLE Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia Chen Sun*,1, Lisbeth Tranebjñrg*,1, Torberg Torbergsen2,GoÈsta Holmgren3 and Marijke Van Ghelue1,4 1Department of Medical Genetics, University Hospital of Tromsù, Tromsù, Norway; 2Department of Neurology, University Hospital of Tromsù, Tromsù, Norway; 3Department of Clinical Genetics, University Hospital of UmeaÊ, UmeaÊ,Sweden;4Department of Biochemistry, Section Molecular Biology, University of Tromsù, Tromsù, Norway Myotonia congenita is a non-dystrophic muscle disorder affecting the excitability of the skeletal muscle membrane. It can be inherited either as an autosomal dominant (Thomsen's myotonia) or an autosomal recessive (Becker's myotonia) trait. Both types are characterised by myotonia (muscle stiffness) and muscular hypertrophy, and are caused by mutations in the muscle chloride channel gene, CLCN1. At least 50 different CLCN1 mutations have been described worldwide, but in many studies only about half of the patients showed mutations in CLCN1. Limitations in the mutation detection methods and genetic heterogeneity might be explanations. In the current study, we sequenced the entire CLCN1 gene in 15 Northern Norwegian and three Northern Swedish MC families. Our data show a high prevalence of myotonia congenita in Northern Norway similar to Northern Finland, but with a much higher degree of mutation heterogeneity. In total, eight different mutations and three polymorphisms (T87T, D718D, and P727L) were detected. Three mutations (F287S, A331T, and 2284+5C4T) were novel while the others (IVS1+3A4T, 979G4A, F413C, A531V, and R894X) have been reported previously. -

Difference in Allelic Expression of the CLCN1 Gene and the Possible Influence on the Myotonia Congenita Phenotype
European Journal of Human Genetics (2004) 12, 738–743 & 2004 Nature Publishing Group All rights reserved 1018-4813/04 $30.00 www.nature.com/ejhg ARTICLE Difference in allelic expression of the CLCN1 gene and the possible influence on the myotonia congenita phenotype Morten Dun1*, Eskild Colding-Jrgensen2, Morten Grunnet3,5, Thomas Jespersen3, John Vissing4 and Marianne Schwartz1 1Department of Clinical Genetics, 4062, University Hospital, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; 2Department of Clinical Neurophysiology 3063,University Hospital, Rigshospitalet, Blegdamsvej 9, DK- 2100 Copenhagen, Denmark; 3Department of Medical Physiology, The Panum Institute, University of Copenhagen, Blegdamsvej 3, DK-2200 Copenhagen N, Denmark; 4Department of Neurology and The Copenhagen Muscle Research Center, University Hospital, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark Mutations in the CLCN1 gene, encoding a muscle-specific chloride channel, can cause either recessive or dominant myotonia congenita (MC). The recessive form, Becker’s myotonia, is believed to be caused by two loss-of-function mutations, whereas the dominant form, Thomsen’s myotonia, is assumed to be a consequence of a dominant-negative effect. However, a subset of CLCN1 mutations can cause both recessive and dominant MC. We have identified two recessive and two dominant MC families segregating the common R894X mutation. Real-time quantitative RT-PCR did not reveal any obvious association between the total CLCN1 mRNA level in muscle and the mode of inheritance, but the dominant family with the most severe phenotype expressed twice the expected amount of the R894X mRNA allele. Variation in allelic expression has not previously been described for CLCN1, and our finding suggests that allelic variation may be an important modifier of disease progression in myotonia congenita. -

Muscle Ion Channel Diseases Rehabilitation Article
ISSN 1473-9348 Volume 3 Issue 1 March/April 2003 ACNR Advances in Clinical Neuroscience & Rehabilitation journal reviews • events • management topic • industry news • rehabilitation topic Review Articles: Looking at protein misfolding neurodegenerative disease through retinitis pigmentosa; Neurological complications of Behçet’s syndrome Management Topic: Muscle ion channel diseases Rehabilitation Article: Domiciliary ventilation in neuromuscular disorders - when and how? WIN BOOKS: See page 5 for details www.acnr.co.uk COPAXONE® WORKS, DAY AFTER DAY, MONTH AFTER MONTH,YEAR AFTER YEAR Disease modifying therapy for relapsing-remitting multiple sclerosis Reduces relapse rates1 Maintains efficacy in the long-term1 Unique MS specific mode of action2 Reduces disease activity and burden of disease3 Well-tolerated, encourages long-term compliance1 (glatiramer acetate) Confidence in the future COPAXONE AUTOJECT2 AVAILABLE For further information, contact Teva Pharmaceuticals Ltd Tel: 01296 719768 email: [email protected] COPAXONE® (glatiramer acetate) PRESCRIBING INFORMATION Presentation Editorial Board and contributors Glatiramer acetate 20mg powder for solution with water for injection. Indication Roger Barker is co-editor in chief of Advances in Clinical Reduction of frequency of relapses in relapsing-remitting multiple Neuroscience & Rehabilitation (ACNR), and is Honorary sclerosis in ambulatory patients who have had at least two relapses in Consultant in Neurology at The Cambridge Centre for Brain Repair. He trained in neurology at Cambridge and at the the preceding two years before initiation of therapy. National Hospital in London. His main area of research is into Dosage and administration neurodegenerative and movement disorders, in particular 20mg of glatiramer acetate in 1 ml water for injection, administered sub- parkinson's and Huntington's disease. -
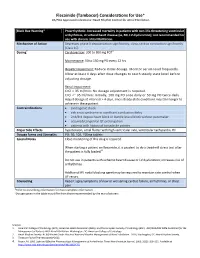
Flecainide Considerations For
Flecainide (Tambocor) Considerations for Use* US/FDA Approved Indications: Heart Rhythm Control for Atrial Fibrillation Black Box Warning* Proarrhythmic. Increased mortality in patients with non-life-threatening ventricular arrhythmias, structural heart disease (ie, MI, LV dysfunction); not recommended for use with chronic atrial fibrillation. Mechanism of Action Depresses phase 0 depolarization significantly, slows cardiac conduction significantly (Class 1C). Dosing† Cardioversion: 200 to 300 mg PO‡1 Maintenance: 50 to 150 mg PO every 12 hrs Hepatic Impairment: Reduce initial dosage. Monitor serum level frequently. Allow at least 4 days after dose changes to reach steady state level before adjusting dosage. Renal Impairment: CrCl > 35 ml/min: No dosage adjustment is required. CrCl <= 35 ml/min: Initially, 100 mg PO once daily or 50 mg PO twice daily. Adjust dosage at intervals > 4 days, since steady-state conditions may take longer to achieve in these patient Contraindications cardiogenic shock sick sinus syndrome or significant conduction delay 2nd/3rd degree heart block or bundle brand block without pacemaker acquired/congenital QT prolongation patients with history of torsade de pointes Major Side Effects hypotension, atrial flutter with high ventricular rate, ventricular tachycardia, HF Dosage forms and Strengths PO: 50, 100, 150mg tablets Special Notes Close monitoring of this drug is required. When starting a patient on flecainide, it is prudent to do a treadmill stress test after the patient is fully loaded.4 Do not use in patients with ischemic heart disease or LV dysfunction; increases risk of arrhythmias. Additional AV nodal blocking agent may be required to maintain rate control when AF recurs. -

A Proposal for the Clinical Use of Flecainide
A Proposal for the Clinical Use of Flecainide JEFFREY L. ANDERSON, MD, JAMES R. STEWART, MD, and BARRY J. CREVEY, MD Effective antiarrhythmic therapy requires a carefully sponse rate is observed in preventing induction of considered approach, including an understanding sustained ventricular tachycardia, and these pa- of the arrhythmia, the underlying cardiac disease tients should be carefully selected. Flecainide is and the drug’s pharmacokinetics. Flecainide is a promising in the treatment of supraventricular new antiarrhythmic drug that may soon be released tachycardias using atrioventricular nodal or extra- for general use. Flecainide demonstrates unsur- nodal reentrant pathways, although this use is still passed efficacy in chronic ventricular arrhythmias investigational in the United States. The drug’s use in stable patients and may become a first-choice for arrhythmias during acute myocardial infarction drug because of its ease of administration, efficacy requires further study. Flecainide possesses modest and favorable tolerance. Twice-daily dosing with negative inotropic potential. Proarrhythmic or other 100 to 200 mg usually provides effective therapy. adverse reactions have occurred primarily in set- Clinical experience suggests flecainide to be indi- tings of high drug level, poor ventricular function cated in the treatment of uniform and multiform or refractory, malignant arrhythmias, suggesting ventricular premature complexes, coupled ven- caution in these groups. tricular premature complexes, and episodes of nonsustained -

Guideline for Preoperative Medication Management
Guideline: Preoperative Medication Management Guideline for Preoperative Medication Management Purpose of Guideline: To provide guidance to physicians, advanced practice providers (APPs), pharmacists, and nurses regarding medication management in the preoperative setting. Background: Appropriate perioperative medication management is essential to ensure positive surgical outcomes and prevent medication misadventures.1 Results from a prospective analysis of 1,025 patients admitted to a general surgical unit concluded that patients on at least one medication for a chronic disease are 2.7 times more likely to experience surgical complications compared with those not taking any medications. As the aging population requires more medication use and the availability of various nonprescription medications continues to increase, so does the risk of polypharmacy and the need for perioperative medication guidance.2 There are no well-designed trials to support evidence-based recommendations for perioperative medication management; however, general principles and best practice approaches are available. General considerations for perioperative medication management include a thorough medication history, understanding of the medication pharmacokinetics and potential for withdrawal symptoms, understanding the risks associated with the surgical procedure and the risks of medication discontinuation based on the intended indication. Clinical judgement must be exercised, especially if medication pharmacokinetics are not predictable or there are significant risks associated with inappropriate medication withdrawal (eg, tolerance) or continuation (eg, postsurgical infection).2 Clinical Assessment: Prior to instructing the patient on preoperative medication management, completion of a thorough medication history is recommended – including all information on prescription medications, over-the-counter medications, “as needed” medications, vitamins, supplements, and herbal medications. Allergies should also be verified and documented. -

Second Generation Antiarrhythmic Agents: Have We Reached Antiarrhythmic Nirvana?
JACC Vol. 9. NO.2 459 February 1987:459-63 EDITORIAL REVIEWS Second Generation Antiarrhythmic Agents: Have We Reached Antiarrhythmic Nirvana? LEONARD N. HOROWITZ, MD, FACC, JOEL MORGANROTH, MD, FACC Philadelphia, Pennsylvania During the first half of the 20th century, antiarrhythmic some cases novel, but because their preapproval evaluations therapy was principally directed toward supraventricular ar followed a more rigorous and circumspect path. More in rhythmia; only relatively recently has therapy of ventricular formation is available to us so we can decide when and how arrhythmias been emphasized. In fact, significant pharma these agents should be employed. cologic treatment of ventricular arrhythmias was minimal Like all antiarrhythmic drugs of the first generation, the until routine use of quinidine and procainamide began in new agents have the potential to provoke or worsen ven the 1950s (1,2). The first generation oral antiarrhythmic tricular arrhythmias. These proarrhythmic effects vary in drugs, quinidine, procainamide and disopyramide, previ incidence and may present a major problem in certain patient ously constituted the principal antiarrhythmic agents for long groups such as those with sustained ventricular tachyar term treatment of ventricular arrhythmias in the United States. rhythrnias, reduced left ventricular function and conduction Compared with modem regulatory standards, the data on disturbances. In common with other antiarrhythmic drugs, which the use of these drugs was based were modest at best the second generation drugs have not been shown to prevent and rudimentary at worst. However, because good clinical sudden death in patients with ventricular ectopic activity. judgment compensates for a multitude of deficiencies, we These factors, along with efficacy and potential toxicity, have been able to provide effective antiarrhythmic therapy must be considered in selecting antiarrhythmic regimens for to many patients with this rather limited pharmacopoeia. -

Skeletal Muscle Channelopathies: a Guide to Diagnosis and Management
Review Pract Neurol: first published as 10.1136/practneurol-2020-002576 on 9 February 2021. Downloaded from Skeletal muscle channelopathies: a guide to diagnosis and management Emma Matthews ,1,2 Sarah Holmes,3 Doreen Fialho2,3,4 1Atkinson- Morley ABSTRACT in the case of myotonia may be precipi- Neuromuscular Centre, St Skeletal muscle channelopathies are a group tated by sudden or initial movement, George's University Hospitals NHS Foundation Trust, London, of rare episodic genetic disorders comprising leading to falls and injury. Symptoms are UK the periodic paralyses and the non- dystrophic also exacerbated by prolonged rest, espe- 2 Department of Neuromuscular myotonias. They may cause significant morbidity, cially after preceding physical activity, and Diseases, UCL, Institute of limit vocational opportunities, be socially changes in environmental temperature.4 Neurology, London, UK 3Queen Square Centre for embarrassing, and sometimes are associated Leg muscle myotonia can cause particular Neuromuscular Diseases, with sudden cardiac death. The diagnosis is problems on public transport, with falls National Hospital for Neurology often hampered by symptoms that patients may caused by the vehicle stopping abruptly and Neurosurgery, London, UK 4Department of Clinical find difficult to describe, a normal examination or missing a destination through being Neurophysiology, King's College in the absence of symptoms, and the need unable to rise and exit quickly enough. Hospital NHS Foundation Trust, to interpret numerous tests that may be These difficulties can limit independence, London, UK normal or abnormal. However, the symptoms social activity, choice of employment Correspondence to respond very well to holistic management and (based on ability both to travel to the Dr Emma Matthews, Atkinson- pharmacological treatment, with great benefit to location and to perform certain tasks) and Morley Neuromuscular Centre, quality of life. -

Severe Infantile Hyperkalaemic Periodic Paralysis And
1339 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.9.1339 on 21 August 2003. Downloaded from SHORT REPORT Severe infantile hyperkalaemic periodic paralysis and paramyotonia congenita: broadening the clinical spectrum associated with the T704M mutation in SCN4A F Brancati, E M Valente, N P Davies, A Sarkozy, M G Sweeney, M LoMonaco, A Pizzuti, M G Hanna, B Dallapiccola ............................................................................................................................. J Neurol Neurosurg Psychiatry 2003;74:1339–1341 the face and hand muscles, and paradoxical myotonia. Onset The authors describe an Italian kindred with nine individu- of paramyotonia is usually at birth.2 als affected by hyperkalaemic periodic paralysis associ- HyperPP/PMC shows characteristics of both hyperPP and ated with paramyotonia congenita (hyperPP/PMC). PMC with varying degrees of overlap and has been reported in Periodic paralysis was particularly severe, with several association with eight mutations in SCN4A gene (I693T, episodes a day lasting for hours. The onset of episodes T704M, A1156T, T1313M, M1360V, M1370V, R1448C, was unusually early, beginning in the first year of life and M1592V).3–9 While T704M is an important cause of isolated persisting into adult life. The paralytic episodes were hyperPP, this mutation has been only recently described in a refractory to treatment. Patients described minimal single hyperPP/PMC family. As with other SCN4A mutations, paramyotonia, mainly of the eyelids and hands. All there can be marked intrafamilial and interfamilial variability affected family members carried the threonine to in paralytic attack frequency and severity in patients harbour- methionine substitution at codon 704 (T704M) in exon 13 ing T704M.10–12 We report an Italian kindred, in which all of the skeletal muscle voltage gated sodium channel gene patients presented with an unusually severe and homogene- (SCN4A). -

Drug Interaction of Amiodarone, Flecainide, Metoprolol and Diltiazem Leading to Heart Block Vijay Kahajuria, Sanjeev Gupta, Roshi, Neelam Rani
JK SCIENCE CASE REPORT Drug Interaction of Amiodarone, Flecainide, Metoprolol and Diltiazem leading to Heart Block Vijay Kahajuria, Sanjeev Gupta, Roshi, Neelam Rani Abstract Amiodarone, flecainide, metoprolol and diltiazem individually are known to cause heart blocks due to their cardiac depressant property but the current case report is worth reporting because it resulted because of drug interaction of multiple drugs due to possible medication error. Key Words Amiodarone, Flecainide, Metoprolol, Diltiazem, Heart Blocks Introduction Adverse Drug Reactions (ADRs) in cardiology are thyroid dysfunction. His hemoglobin levels were 13g/dl, common. There are numerous reports of drug induced total leucocyte count 6000/UL, neutrophils 56.5%, bradycardia and heart block (1). Co-morbid conditions lymphocytes 30.5%, eosinophils 5.2%, monocytes often coexist with cardiac ailments and results in 7.6%,basophils 0.2%, platelet count 1.5 lacs, serum urea- polypharmacy which enhance the chances of drug 33mg/dl, serum creatinine 0.90mg/dl, prothrombin time interactions culminating to adverse events. In the current 10.9 sec ,INR 1.04,hepatitis serology was non reactive, study report patient was prescribed multiple drugs for blood sugar random 182 mg/dl, TSH 1.4uIU/ml, serum cardiac arrhythmia and he presented with heart block sodium 142 mmol/L,serum potassium 5 mmol/L. and bradycardia. Though amiodarone, flecainide, He was prescribed tab.atenolol 20mg and metoprolol and diltiazem individually are known to cause tab.procainamide 20mg which did not bring any relief. heart blocks due to their cardiac depressant property but So radiofrequency ablation was done which was the current case report is worth reporting because it uneventful. -

Formulation Development Strategies
FORMULATION DEVELOPMENT STRATEGIES FOR ORAL EXTENDED RELEASE DOSAGE FORMS Dissertation zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften (Dr. rer. nat.) eingereicht im Fachbereich Biologie, Chemie, Pharmazie der Freien Universität Berlin vorgelegt von ARAYA RAIWA aus Bangkok, Thailand May, 2011 1. Gutachter: Prof. Dr. Roland Bodmeier 2. Gutachter: Prof. Dr. Jürgen Siepmann Disputation am 9.Juni 2011 TO MY FAMILY ACKNOWLEDGEMENTS First and foremost, I wish to express my deepest gratitude to my supervisor, Prof. Dr. Roland Bodmeier for his professional guidance, helpful advices and encouragement. I am very grateful for his scientific and financial support and for providing me such an interesting topic. Furthermore, I am very thankful to him for the opportunity to support his editorial role in the European Journal of Pharmaceutical Sciences. I would like to thank Prof. Dr. Jürgen Siepmann for co-evaluating this thesis. Thanks are extended to Prof. Dr. Herbert Kolodziej, Prof. Dr. Johannes Peter Surmann and Dr. Martin Körber for serving as members of my thesis advisor committee. I am particular thankful to Dr. Andrei Dashevsky, Dr. Nantharat Pearnchob and Dr. Martin Körber for their very useful discussion; Dr. Burkhard Dickenhorst for evaluating parts of this thesis; Mrs. Angelika Schwarz for her assistance with administrative issues; Mr. Andreas Krause, Mrs. Eva Ewest and Mr. Stefan Walter for the prompt and diligent technical support. Sincere thanks are extended to Dr. Ildiko Terebesi, Dr. Burkhard Dickenhorst, Dr. Soravoot Rujivipat and Dr. Samar El-Samaligy for the friendly atmosphere in the lab My special thanks are owing to all members from the Kelchstrasse for their practical advice, enjoyable discussion and kindness throughout the years.