Levosimendan in Patients with Left Ventricular Dysfunction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharmaceutical Services Division and the Clinical Research Centre Ministry of Health Malaysia
A publication of the PHARMACEUTICAL SERVICES DIVISION AND THE CLINICAL RESEARCH CENTRE MINISTRY OF HEALTH MALAYSIA MALAYSIAN STATISTICS ON MEDICINES 2008 Edited by: Lian L.M., Kamarudin A., Siti Fauziah A., Nik Nor Aklima N.O., Norazida A.R. With contributions from: Hafizh A.A., Lim J.Y., Hoo L.P., Faridah Aryani M.Y., Sheamini S., Rosliza L., Fatimah A.R., Nour Hanah O., Rosaida M.S., Muhammad Radzi A.H., Raman M., Tee H.P., Ooi B.P., Shamsiah S., Tan H.P.M., Jayaram M., Masni M., Sri Wahyu T., Muhammad Yazid J., Norafidah I., Nurkhodrulnada M.L., Letchumanan G.R.R., Mastura I., Yong S.L., Mohamed Noor R., Daphne G., Kamarudin A., Chang K.M., Goh A.S., Sinari S., Bee P.C., Lim Y.S., Wong S.P., Chang K.M., Goh A.S., Sinari S., Bee P.C., Lim Y.S., Wong S.P., Omar I., Zoriah A., Fong Y.Y.A., Nusaibah A.R., Feisul Idzwan M., Ghazali A.K., Hooi L.S., Khoo E.M., Sunita B., Nurul Suhaida B.,Wan Azman W.A., Liew H.B., Kong S.H., Haarathi C., Nirmala J., Sim K.H., Azura M.A., Asmah J., Chan L.C., Choon S.E., Chang S.Y., Roshidah B., Ravindran J., Nik Mohd Nasri N.I., Ghazali I., Wan Abu Bakar Y., Wan Hamilton W.H., Ravichandran J., Zaridah S., Wan Zahanim W.Y., Kannappan P., Intan Shafina S., Tan A.L., Rohan Malek J., Selvalingam S., Lei C.M.C., Ching S.L., Zanariah H., Lim P.C., Hong Y.H.J., Tan T.B.A., Sim L.H.B, Long K.N., Sameerah S.A.R., Lai M.L.J., Rahela A.K., Azura D., Ibtisam M.N., Voon F.K., Nor Saleha I.T., Tajunisah M.E., Wan Nazuha W.R., Wong H.S., Rosnawati Y., Ong S.G., Syazzana D., Puteri Juanita Z., Mohd. -

Estonian Statistics on Medicines 2016 1/41
Estonian Statistics on Medicines 2016 ATC code ATC group / Active substance (rout of admin.) Quantity sold Unit DDD Unit DDD/1000/ day A ALIMENTARY TRACT AND METABOLISM 167,8985 A01 STOMATOLOGICAL PREPARATIONS 0,0738 A01A STOMATOLOGICAL PREPARATIONS 0,0738 A01AB Antiinfectives and antiseptics for local oral treatment 0,0738 A01AB09 Miconazole (O) 7088 g 0,2 g 0,0738 A01AB12 Hexetidine (O) 1951200 ml A01AB81 Neomycin+ Benzocaine (dental) 30200 pieces A01AB82 Demeclocycline+ Triamcinolone (dental) 680 g A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+ Thymol (dental) 3094 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+ Cetylpyridinium chloride (gingival) 227150 g A01AD81 Lidocaine+ Cetrimide (O) 30900 g A01AD82 Choline salicylate (O) 864720 pieces A01AD83 Lidocaine+ Chamomille extract (O) 370080 g A01AD90 Lidocaine+ Paraformaldehyde (dental) 405 g A02 DRUGS FOR ACID RELATED DISORDERS 47,1312 A02A ANTACIDS 1,0133 Combinations and complexes of aluminium, calcium and A02AD 1,0133 magnesium compounds A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 811120 pieces 10 pieces 0,1689 A02AD81 Aluminium hydroxide+ Magnesium hydroxide (O) 3101974 ml 50 ml 0,1292 A02AD83 Calcium carbonate+ Magnesium carbonate (O) 3434232 pieces 10 pieces 0,7152 DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 46,1179 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 2,3855 A02BA02 Ranitidine (O) 340327,5 g 0,3 g 2,3624 A02BA02 Ranitidine (P) 3318,25 g 0,3 g 0,0230 A02BC Proton pump inhibitors 43,7324 A02BC01 Omeprazole -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Simdax® Gives You Time in Acute Heart Failure
SIMDAX ® GIVES YOU TIME IN ACUTE HEART FAILURE 1 SIMDAX® RELIEVES Improvement in clinical status Improved SYMPTOMS 20 p=0.015 19.4% 14.6% Levosimendan + SOC (n=299) IN AHF 10 Placebo + SOC (n=301) p=0.015 SIMDAX® improves symptoms of dyspnoea 0 and fatigue in acute heart failure. In the The primary endpoint result in the -10 REVIVE trial1, composite consisting Phase III regulatory study REVIVE, symptoms of patients’ subjective symptom -19.4% assessments (at 6 hours, 24 hours, over the 5-day assessment period improved -20 and 5 days) and signs of worsening -27.2% symptoms (including death) during significantly more with SIMDAX® than with Patients improved or worsened (%) the 5 days after starting drug -30 Worsened infusion. placebo when administered on top of the standard of care.1 Improvement in dyspnea 100 Generalized linear model p=0.018 Levosimendan + SOC (n=299) 90 Placebo + SOC (n=301) 80 Effects of levosimendan vs 70 placebo on top of standard of care on dyspnea in patients with AHF.4 60 Patients improved (%)* 6 hrs 24 hrs 48 hrs Day 3 Day 5 Reference: 1. Packer M. et al. JCHF. 2013;1(2): 103-11. *Includes mild, moderate and marked improvement on a 7-point scale 2 …WITH SUSTAINED HEMODYNAMIC AND Levosimendan + SOC (n=299) NEUROHORMONAL EFFECTS Placebo + SOC (n=301) The hemodynamic effects of SIMDAX® Sustained hemodynamic effects on cardiac output (CO) and pulmonary 1 CO (L/min) Max capillary wedge pressure (PCWP) have been End PCWP (mmHg) 0 1-3 24-hr infusion Figure 1: Duration of action of shown in several clinical trials. -

Estonian Statistics on Medicines 2013 1/44
Estonian Statistics on Medicines 2013 DDD/1000/ ATC code ATC group / INN (rout of admin.) Quantity sold Unit DDD Unit day A ALIMENTARY TRACT AND METABOLISM 146,8152 A01 STOMATOLOGICAL PREPARATIONS 0,0760 A01A STOMATOLOGICAL PREPARATIONS 0,0760 A01AB Antiinfectives and antiseptics for local oral treatment 0,0760 A01AB09 Miconazole(O) 7139,2 g 0,2 g 0,0760 A01AB12 Hexetidine(O) 1541120 ml A01AB81 Neomycin+Benzocaine(C) 23900 pieces A01AC Corticosteroids for local oral treatment A01AC81 Dexamethasone+Thymol(dental) 2639 ml A01AD Other agents for local oral treatment A01AD80 Lidocaine+Cetylpyridinium chloride(gingival) 179340 g A01AD81 Lidocaine+Cetrimide(O) 23565 g A01AD82 Choline salicylate(O) 824240 pieces A01AD83 Lidocaine+Chamomille extract(O) 317140 g A01AD86 Lidocaine+Eugenol(gingival) 1128 g A02 DRUGS FOR ACID RELATED DISORDERS 35,6598 A02A ANTACIDS 0,9596 Combinations and complexes of aluminium, calcium and A02AD 0,9596 magnesium compounds A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 591680 pieces 10 pieces 0,1261 A02AD81 Aluminium hydroxide+Magnesium hydroxide(O) 1998558 ml 50 ml 0,0852 A02AD82 Aluminium aminoacetate+Magnesium oxide(O) 463540 pieces 10 pieces 0,0988 A02AD83 Calcium carbonate+Magnesium carbonate(O) 3049560 pieces 10 pieces 0,6497 A02AF Antacids with antiflatulents Aluminium hydroxide+Magnesium A02AF80 1000790 ml hydroxide+Simeticone(O) DRUGS FOR PEPTIC ULCER AND GASTRO- A02B 34,7001 OESOPHAGEAL REFLUX DISEASE (GORD) A02BA H2-receptor antagonists 3,5364 A02BA02 Ranitidine(O) 494352,3 g 0,3 g 3,5106 A02BA02 Ranitidine(P) -

Vasopressors and Inotropes in Acute Myocardial Infarction Related Cardiogenic Shock: a Systematic Review and Meta-Analysis
Journal of Clinical Medicine Review Vasopressors and Inotropes in Acute Myocardial Infarction Related Cardiogenic Shock: A Systematic Review and Meta-Analysis 1, 2, 1 3 Mina Karami y , Veemal V. Hemradj y, Dagmar M. Ouweneel , Corstiaan A. den Uil , Jacqueline Limpens 4, Luuk C. Otterspoor 5, Alexander P. Vlaar 6 , Wim K. Lagrand 6 and José P. S. Henriques 1,* 1 Heart Center, Department of Interventional Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam UMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] (M.K.); [email protected] (D.M.O.) 2 Department of Cardiology, Isala, 8025 AB Zwolle, The Netherlands; [email protected] 3 Departments of Cardiology and Intensive Care Medicine, Erasmus MC, University Medical Center, 3015 GD Rotterdam, The Netherlands; [email protected] 4 Medical Library, Amsterdam UMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] 5 Heart Center Catharina Hospital, 5623 EJ Eindhoven, The Netherlands; [email protected] 6 Department of Intensive Care, Amsterdam UMC, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] (A.P.V.); [email protected] (W.K.L.) * Correspondence: [email protected] These two authors contributed equally. y Received: 5 May 2020; Accepted: 25 June 2020; Published: 30 June 2020 Abstract: Vasopressors and inotropes are routinely used in acute myocardial infarction (AMI) related cardiogenic shock (CS) to improve hemodynamics. We aimed to investigate the effect of routinely used vasopressor and inotropes on mortality in AMI related CS. A systematic search of MEDLINE, EMBASE and CENTRAL was performed up to 20 February 2019. -

Study Protocol
Product: Omecamtiv Mecarbil (AMG 423) Protocol Number: 20110203 Date: 13 November 2018 Page 1 of 85 Title: A Double-blind, Randomized, Placebo-controlled, Multicenter Study to Assess the Efficacy and Safety of Omecamtiv Mecarbil on Mortality and Morbidity in Subjects With Chronic Heart Failure With Reduced Ejection Fraction Amgen Protocol Number (Omecamtiv Mecarbil [AMG 423]) 20110203 EudraCT number 2016-002299-28 NCT Number NCT02929329 GALACTIC-HF Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure Clinical Study Sponsor: Amgen Inc. One Amgen Center Drive Thousand Oaks, CA 91320-1799 Telephone: 1-805-447-1000 Key Sponsor Contact(s): One Amgen Center Drive Thousand Oaks, CA 91320-1799 Phone: Email: Date: 31 August 2016 Amendment 1 07 September 2017 Amendment 2 13 November 2018 Confidentiality Notice This document contains confidential information of Amgen Inc. Approved This document must not be disclosed to anyone other than the site study staff and members of the institutional review board/independent ethics committee/institutional scientific review board or equivalent. The information in this document cannot be used for any purpose other than the evaluation or conduct of the clinical investigation without the prior written consent of Amgen Inc. If you have questions regarding how this document may be used or shared, call the Amgen Medical Information number: US sites, 1-800-77-AMGEN, Canadian sites, 1-866-50-AMGEN; all other countries call 1-800-772-6436. Amgen’s general number in the US (1-805-447-1000). CONFIDENTIAL Product: Omecamtiv Mecarbil (AMG 423) Protocol Number: 20110203 Date: 13 November 2018 Page 2 of 85 Investigator’s Agreement I have read the attached protocol entitled A Double-blind, Randomized, Placebo-controlled, Multicenter Study to Assess the Efficacy and Safety of Omecamtiv Mecarbil on Mortality and Morbidity in Subjects with Chronic Heart Failure with Reduced Ejection Fraction, dated 13 November 2018, and agree to abide by all provisions set forth therein. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

The Calcium Sensitizer Levosimendan Improves Human Diaphragm Function
Page 1 of 41 The calcium sensitizer levosimendan improves human diaphragm function Jonne Doorduin MSc 1, Christer A Sinderby PhD 5,7 , Jennifer Beck PhD 6,7 , Dick F Stegeman PhD 2,4 , Hieronymus WH van Hees PhD 3, Johannes G van der Hoeven MD 1, and Leo MA Heunks MD 1 1Department of Critical Care Medicine, 2Department of Neurology and 3Department of Pulmonary Diseases, Radboud University Nijmegen Medical Centre, The Netherlands; 4Faculty of Human Movement Sciences, Research Institute MOVE, VU University, Amsterdam, The Netherlands; 5Department of Medicine, Division of Critical Care Medicine, 6Department of Pediatrics, St. Michael’s Hospital, University of Toronto, Canada; 7Keenan Research Centre in the Li Ka Shing Knowledge Institute of St. Michael’s Hospital, University of Toronto, Canada. Address for correspondents and requests: Leo Heunks, MD, PhD Radboud University Nijmegen Medical Centre Department of Critical Care Medicine Geert Grooteplein zuid 10 6525 GA Nijmegen The Netherlands tel. : +31 24 36 17273 fax. : +31 24 35 41612 [email protected] Page 2 of 41 Author’s contributions Literature search: JD, LH; Study design: JD, CS, JB, DS, LH; Data collection: JD, LH; Data analysis: JD, LH; Data interpretation: JD, CS, JB, DS, HH, JH, LH; Writing: JD, CS, JB, DS, HH, JH, LH. Source of funding This study was investigator initiated and financed by institutional resources. This study was designed and conducted by the authors without involvement of Orion Pharma, or any other commercial party. Data analysis and writing the manuscript was performed by the authors without involvement of a commercial party. Levosimendan and placebo were an unrestricted gift from Orion Pharma (Espoo Finland). -
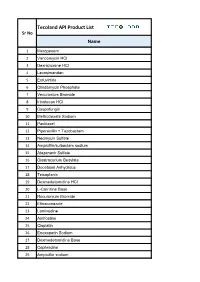
Tecoland API Product List Sr No Name
Tecoland API Product List Sr No Name 1 Meropenem 2 Vancomycin HCl 3 Dexrazoxane HCl 4 Levosimendan 5 Enfuvirtide 6 Clindamycin Phosphate 7 Vecuronium Bromide 8 Irinotecan HCl 9 Caspofungin 10 Methotrexate Sodium 11 Paclitaxel 12 Piperacillin + Tazobactam 13 Neomycin Sulfate 14 Ampicillin/sulbactam sodium 15 Atazanavir Sulfate 16 Cisatracurium Besylate 17 Docetaxel Anhydrous 18 Teicoplanin 19 Dexmedetomidine HCl 20 L-Carnitine Base 21 Rocuronium Bromide 22 Efinaconazole 23 Lamivudine 24 Amifostine 25 Cisplatin 26 Enoxaparin Sodium 27 Dexmedetomidine Base 28 Cephradine 29 Ampicillin sodium Tecoland API Product List Sr No Name 30 Sodium Nitroprusside 31 Ertapenem sodium 32 Loratadine 33 Docetaxel Trihydrate 34 Mitoxantrone Hcl 35 Linezolid 36 Esmolol HCl 37 Cefotaxime sodium 38 Bosentan monohydrate 39 Ezetimibe 40 Sevoflurane 41 Hydrocortisone base 42 Leflunomide 43 Fludarabine phosphate 44 Moxifloxacin HCl 45 Montelukast Sodium 46 Amphotericin B 47 Palonosetron Hcl 48 Ifosfamide 49 Ceftazidime 50 Nitrofurantoin 51 Lincomycin HCl 52 Cephapirin Benzathine 53 Lopinavir 54 Mesna 55 Erlotinib HCl 56 Granisetron HCl 57 Hyaluronate Sodium 58 Temozolomide Tecoland API Product List Sr No Name 59 Pyridoxine HCl 60 Suxamethonium Chloride 61 Tetracycline HCl 62 Hydralazine HCl 63 Glatiramer Acetate 64 Penciclovir 65 Cyclosporine 66 Irbesartan 67 Flumazenil 68 Calcium Folinate 69 Felodipine 70 Anidulafungin 71 cytarabine 72 Travoprost 73 Sugammadex Sodium 74 Oritavancin 75 Milrinone 76 Benzocaine 77 Simvastatin 78 Fosaprepitant dimeglumine 79 Oseltamivir -

AHRQ Healthcare Horizon Scanning System – Status Update Horizon
AHRQ Healthcare Horizon Scanning System – Status Update Horizon Scanning Status Update: July 2014 Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Contract No. HHSA290201000006C Prepared by: ECRI Institute 5200 Butler Pike Plymouth Meeting, PA 19462 July 2014 Statement of Funding and Purpose This report incorporates data collected during implementation of the Agency for Healthcare Research and Quality (AHRQ) Healthcare Horizon Scanning System by ECRI Institute under contract to AHRQ, Rockville, MD (Contract No. HHSA290201000006C). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services. A novel intervention may not appear in this report simply because the System has not yet detected it. The list of novel interventions in the Horizon Scanning Status Update Report will change over time as new information is collected. This should not be construed as either endorsements or rejections of specific interventions. As topics are entered into the System, individual target technology reports are developed for those that appear to be closer to diffusion into practice in the United States. A representative from AHRQ served as a Contracting Officer’s Technical Representative and provided input during the implementation of the horizon scanning system. AHRQ did not directly participate in the horizon scanning, assessing the leads or topics, or provide opinions regarding potential impact of interventions. -

Levosimendan Is Superior to Enoximone in Refractory Cardiogenic Shock Complicating Acute Myocardial Infarction*
Clinical Investigations Levosimendan is superior to enoximone in refractory cardiogenic shock complicating acute myocardial infarction* Joerg T. Fuhrmann, MD; Alexander Schmeisser, MD; Matthias R. Schulze, MD; Carsten Wunderlich, MD; Steffen P. Schoen, MD; Thomas Rauwolf, PhD; Christof Weinbrenner, MD; Ruth H. Strasser, MD Objective: Cardiogenic shock is the leading cause of death in Measurements and main results: Survival rate at 30 days was patients hospitalized for acute myocardial infarction. The objec- significantly higher in the levosimendan-treated group (69%, 11 of ؍ tives were to investigate the effects of levosimendan, a novel 16) compared with the enoximone group (37%, 6 of 16, p inodilator, compared with the phosphodiesterase-III inhibitor 0.023). Invasive hemodynamic parameters during the first 48 hrs enoximone in refractory cardiogenic shock complicating acute were comparable in both groups. Levosimendan induced a trend myocardial infarction, on top of current therapy. toward higher cardiac index, cardiac power index, left ventricular Design: Prospective, randomized, controlled single-center clin- stroke work index, and mixed venous oxygen saturation. In ad- ical trial. dition, lower cumulative values for catecholamines at 72 hrs and Setting: Medical and coronary intensive care unit in a univer- for clinical signs of inflammation were seen in the levosimendan- sity hospital. treated patients. Multiple organ failure leading to death occurred Patients: Thirty-two patients with refractory cardiogenic shock exclusively in the enoximone group (4 of 16 patients). for at least 2 hrs requiring additional therapy. Conclusions: In severe and refractory cardiogenic shock com- Interventions: Infusion of either levosimendan (12 g/kg over plicating acute myocardial infarction, levosimendan, added to 10 min, followed by 0.1 g/kg/min over 50 min, and of 0.2 current therapy, may contribute to improved survival compared g/kg/min for the next 23 hrs) or enoximone (fractional loading with enoximone.