Failure to Thrive
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CHALLENGING CASES: BIOPSYCHOSOCIAL PEDIATRICS Failure to Thrive in a 4-Month-Old Nursing Infant
CHALLENGING CASES: BIOPSYCHOSOCIAL PEDIATRICS Failure to Thrive in a 4-Month-Old Nursing Infant* CASE visits she was observed to respond quickly to visual Christine, a 4-month-old infant, is brought to the and auditory cues. There was no family history of office for a health-supervision visit by her mother, serious diseases. a 30-year-old emergency department nurse. The In that a comprehensive history and physical ex- mother, who is well known to the pediatrician to be amination did not indicate an organic cause, the a competent and attentive caregiver, appears unchar- pediatrician reasoned that the most likely cause of acteristically tired. They are accompanied by the 18- her failure to thrive was an unintentional caloric month-old and 4-year-old siblings because child care deprivation secondary to maternal stress and ex- was unavailable, and her husband has been out of haustion. Christine’s mother and the pediatrician town on business for the last 5 weeks. When the discussed how maternal stress, fatigue, and depres- pediatrician comments, “You look exhausted. It must sion could hinder the let-down reflex and reduce the be really tough to care for 3 young children,” the availability of an adequate milk supply, as well as mother appears relieved that attention was given to make it difficult to maintain regular feeding sessions. her needs. She reports that she has not been getting The pediatrician recommended obtaining help with much sleep because Christine wakes up for 2 or more child care and household responsibilities. The evening feedings and the toddler is now awakening mother was also encouraged to consume adequate at night. -

A Dose-Response Relationship Between Organic Mercury Exposure from Thimerosal-Containing Vaccines and Neurodevelopmental Disorders
Int. J. Environ. Res. Public Health 2014, 11, 9156-9170; doi:10.3390/ijerph110909156 OPEN ACCESS International Journal of Environmental Research and Public Health ISSN 1660-4601 www.mdpi.com/journal/ijerph Article A Dose-Response Relationship between Organic Mercury Exposure from Thimerosal-Containing Vaccines and Neurodevelopmental Disorders David A. Geier 1, Brian S. Hooker 2, Janet K. Kern 1,3, Paul G. King 4, Lisa K. Sykes 4 and Mark R. Geier 1,* 1 Institute of Chronic Illnesses, Inc., 14 Redgate Ct., Silver Spring, MD 20905, USA; E-Mails: [email protected] (D.A.G.); [email protected] (J.K.K.) 2 Department of Biology, Simpson University, 2211 College View Dr., Redding, CA 96003, USA; E-Mail: [email protected] 3 Department of Psychiatry, University of Texas Southwestern Medical Center at Dallas, 5353 Harry Hine Blvd., Dallas, TX 75390, USA 4 CoMeD, Inc., 14 Redgate Ct., Silver Spring, MD 20905, USA; E-Mails: [email protected] (P.G.K.); [email protected] (L.K.S.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-301-989-0548; Fax: +1-301-989-1543. Received: 12 July 2014; in revised form: 7 August 2014 / Accepted: 26 August 2014 / Published: 5 September 2014 Abstract: A hypothesis testing case-control study evaluated concerns about the toxic effects of organic-mercury (Hg) exposure from thimerosal-containing (49.55% Hg by weight) vaccines on the risk of neurodevelopmental disorders (NDs). Automated medical records were examined to identify cases and controls enrolled from their date-of-birth (1991–2000) in the Vaccine Safety Datalink (VSD) project. -

Hypokalaemia in a Woman with Eating Disorder
Grand Rounds Vol 11 pages 53–55 Specialities: Acute Medicine; Nephrology; Psychiatry Article Type: Case Report DOI: 10.1102/1470-5206.2011.0013 ß 2011 e-MED Ltd Hypokalaemia in a woman with eating disorder Zachary Z. Brenera, Boris Medvedovskya, James F. Winchestera and Michael Bergmanb aDivision of Nephrology, Department of Medicine, Beth Israel Medical Center, Albert Einstein School of Medicine of Yeshiva University, New York, USA; bDepartment of Medicine, Campus Golda, Rabin Medical Center, Petah-Tikva, Tel-Aviv University, Israel Corresponding address: Dr Zachary Z. Brener, 350 E. 17th St., Division of Nephrology, Beth Israel Medical Center, New York, NY 10003, USA. Email: [email protected] Date accepted for publication 13 April 2011 Abstract Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. A concealed diuretic abuse should be suspected, especially in young women with eating disorders. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to early and accurate diagnosis. Keywords Hypokalaemia; eating disorders; diuretics. Introduction Chronic hypokalaemia often remains a diagnostic challenge, especially in young women without hypertension. After the exclusion of the most obvious causes, a concealed diuretic abuse associated with or without surreptitious vomiting and laxative abuse should be suspected, especially in young women concerned with their body image. A conclusive diagnosis may be difficult as such patients often vigorously deny diuretic intake[1]. Also, only a minority of patients with eating disorders (approximately 6%) abuse diuretics[2–4]. This case describes a woman with chronic hypokalaemia in whom a thorough medical history and proper laboratory tests were essential to an early and accurate diagnosis. -

Intervention Ideas for Infants, Toddlers, Children, and Youth Impacted by Opioids
TOPICAL ISSUE BRIEF Intervention IDEAs for Infants, Toddlers, Children, and Youth Impacted by Opioids Overview Prevalence The abuse of opioids—such as heroin and various prescription drugs commonly prescribed for pain (e.g., oxycodone, hydrocodone, and fentanyl)—has rapidly gained attention across the United States as a public health crisis. Youth may be exposed to opioids in a variety of ways, including but not limited to infants born to mothers who took opioids during pregnancy; children mistakenly consuming opioids (perhaps thinking they are candy); teenagers taking opioids from an illicit street supply; or, more commonly, teenagers being given opiates for free by a friend or relative.1,2 Even appropriate opioid use among adolescents and young adults to treat pain may slightly increase their risk of later opioid misuse.3 The Centers for Disease Control and Prevention (CDC) have identified opioid abuse as an epidemic, noting that opioids impact and affect all communities and age groups.4 Between 1997 and 2012, 13,052 children were hospitalized for poisonings caused by oxycodone, Percocet (a combination of acetaminophen and oxycodone), codeine, and other prescription opioids.5 Every 25 minutes, an infant is born suffering from opioid withdrawal, and an estimated 21,732 infants were born in 2012 with neonatal abstinence syndrome (NAS), a drug withdrawal syndrome.6 According to data from 2014, approximately 28,000 adolescents had used heroin within the past year, 16,000 were current heroin users, and 18,000 had a heroin use disorder.7 At the same time, relatively few data are available about the effects of opiates on the U.S. -

Child and Adolescent Needs and Strengths: Early Childhood
Child and Adolescent Needs and Strengths: Early Childhood CANS: EC Supplemental Guide A Support for the CANS (Early Childhood) Information Integration Tool for Young Children Ages Birth to Five SET is part of the System of Care Initiative, within the Allegheny County Department of Human Services, Office of Behavioral Health Stacey Cornett, Copyright 2007. Child and Adolescent Needs and Strengths: Early Childhood (CANS:EC) Section One: Child Strengths This section focuses on the attributes, traits, talents and skills of the child that can be useful in developing a strength-based treatment plan. Within a systems of care approach identifying strengths is considered an essential part of the process (Miles, P., Burns, E.J., Osher, T.W., Walker, J.S., 2006). A focus on strengths that both the child and the family have to offer allows for a climate of hope and optimism and has been proven to engage families at a higher level. Strength-based assessment has been defined as, “the measurement of those emotional and behavioral skills, competencies, and characteristics that create a sense of personal accomplishment; contribute to satisfying relationships with family members, peers, and adults; enhance one’s ability to deal with adversity and stress; and promote one’s personal, social, and academic development” (Epstein & Sharma, 1998, p.3). The benefits of a strength- based assessment include involving parents and children in a service planning experience that builds on what a child and family are doing well, facilitates positive expectations for the child, and empowers family members to take responsibility for the decisions that will affect their child (Johnson & Friedman, 1991; Saleebey, 1992). -

Magnesium: the Forgotten Electrolyte—A Review on Hypomagnesemia
medical sciences Review Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia Faheemuddin Ahmed 1,* and Abdul Mohammed 2 1 OSF Saint Anthony Medical Center, 5666 E State St, Rockford, IL 61108, USA 2 Advocate Illinois Masonic Medical Center, 833 W Wellington Ave, Chicago, IL 60657, USA; [email protected] * Correspondence: [email protected] Received: 20 February 2019; Accepted: 2 April 2019; Published: 4 April 2019 Abstract: Magnesium is the fourth most abundant cation in the body and the second most abundant intracellular cation. It plays an important role in different organ systems at the cellular and enzymatic levels. Despite its importance, it still has not received the needed attention either in the medical literature or in clinical practice in comparison to other electrolytes like sodium, potassium, and calcium. Hypomagnesemia can lead to many clinical manifestations with some being life-threatening. The reported incidence is less likely than expected in the general population. We present a comprehensive review of different aspects of magnesium physiology and hypomagnesemia which can help clinicians in understanding, identifying, and treating this disorder. Keywords: magnesium; proton pump inhibitors; diuretics; hypomagnesemia 1. Introduction Magnesium is one of the most abundant cation in the body as well as an abundant intracellular cation. It plays an important role in molecular, biochemical, physiological, and pharmacological functions in the body. The importance of magnesium is well known, but still it is the forgotten electrolyte. The reason for it not getting the needed attention is because of rare symptomatology until levels are really low and also because of a lack of proper understanding of magnesium physiology. -

The Child with Failure to Thrive
The Child with Failure to Thrive Bonnie Proulx, MSN, APRN, PNP Pediatric Gastroenterology Children’s Hospital at Dartmouth Manchester, NH DISCLOSURES None of the planners or presenters of this session have disclosed any conflict or commercial interest The Child with Failure to Thrive OBJECTIVES: 1. Review assessment and appropriate diagnostic testing for children who fail meet expected growth 2. Discuss various causes of failure to thrive. 3. Identify appropriate referrals for children with failure to thrive. Definition • Inadequate physical growth diagnosed by observation of growth over time on a standard growth chart Definition • More than 2 standard deviations below mean for age • Downward trend in growth crossing 2 major percentiles in a short time • Comparison of height and weight • Not a diagnosis but a finding Pitfalls • Some children are just tiny • Familial short stature children may cross percentiles to follow genetic predisposition • Normal small children may be diagnosed later given already tiny Frequency • Affects 5-10% of children under 5 in developing countries • Peak incidence between 9 and 24 months of age • Uncommon after the age of 5 What is normal growth • Birth to 6 months, a baby may grow 1/2 to 1 inch (about 1.5 to 2.5 centimeters) a month • 6 to 12 months, a baby may grow 3/8 inch (about 1 centimeter) a month • 2nd year of life 4-6 inches • 3rd year 3-4 inches • Annual growth until pre-puberty 2-3 inches per year Normal weight gain • Birth to 6 months-gain 5 to 7 ounces a week. • 6 to 12 months -gain 3 to 5 ounces (about 85 to 140 grams) a week. -
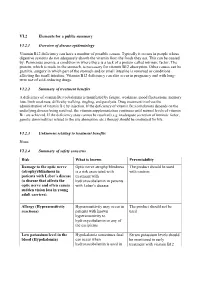
VI.2 Elements for a Public Summary VI.2.1 Overview of Disease Epidemiology Vitamin B12 Deficiency Can Have a Number of Possible
VI.2 Elements for a public summary VI.2.1 Overview of disease epidemiology Vitamin B12 deficiency can have a number of possible causes. Typically it occurs in people whose digestive systems do not adequately absorb the vitamin from the foods they eat. This can be caused by: Pernicious anemia, a condition in where there is a lack of a protein called intrinsic factor. The protein, which is made in the stomach, is necessary for vitamin B12 absorption. Other causes can be gastritis, surgery in which part of the stomach and/or small intestine is removed or conditions affecting the small intestine. Vitamin B12 deficiency can also occur in pregnancy and with long- term use of acid-reducing drugs. VI.2.2 Summary of treatment benefits A deficiency of vitamin B12 (cobalamin) is manifested by fatigue, weakness, mood fluctuations, memory loss, limb weakness, difficulty walking, tingling, and paralysis. Drug treatment involves the administration of vitamin B12 by injection. If the deficiency of vitamin B12 (cobalamin) depends on the underlying disease being resolved, the vitamin supplementation continues until normal levels of vitamin B12 are achieved. If the deficiency state cannot be resolved (e.g. inadequate secretion of intrinsic factor, genetic abnormalities related to the site absorption, etc.) therapy should be continued for life. VI.2.3 Unknowns relating to treatment benefits None. VI.2.4 Summary of safety concerns Risk What is known Preventability Damage to the optic nerve Optic nerve atrophy/blindness The product should be used (atrophy)/blindness in is a risk associated with with caution. patients with Leber´s disease treatment with (a disease that affects the hydroxocobalamin in patients optic nerve and often causes with Leber´s disease. -

Wasting and Body Composition of Adults with Pulmonary Tuberculosis in Relation to HIV-1 Coinfection, Socioeconomic Status, and Severity of Tuberculosis
European Journal of Clinical Nutrition (2006) 60, 163–171 & 2006 Nature Publishing Group All rights reserved 0954-3007/06 $30.00 www.nature.com/ejcn ORIGINAL ARTICLE Wasting and body composition of adults with pulmonary tuberculosis in relation to HIV-1 coinfection, socioeconomic status, and severity of tuberculosis E Villamor1, E Saathoff1, F Mugusi2, RJ Bosch3,4, W Urassa5 and WW Fawzi1,6 1Department of Nutrition, Harvard School of Public Health, Boston, MA, USA; 2Department of Internal Medicine, Muhimbili University College of Health Sciences, Dar es Salaam, Tanzania; 3Department of Biostatistics, Harvard School of Public Health, Boston, MA, USA; 4Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, MA, USA; 5Department of Microbiology and Immunology, Muhimbili University College of Health Sciences, Dar es Salaam, Tanzania and 6Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA Objective: To examine the impact of HIV coinfection, socioeconomic status (SES) and severity of tuberculosis (TB) on the body composition and anthropometric status of adults with pulmonary TB. Design: Cross-sectional study. Setting: Five TB clinics in Dar es Salaam, Tanzania. Subjects: A total of 2231 adult men and women diagnosed with pulmonary TB, prior to the initiation of anti-TB therapy. Methods: We compared the distribution of anthropometric characteristics including body mass index (BMI), mid-upper arm circumference (MUAC), triceps skin-fold (TSF), and arm muscle circumference (AMC) by HIV status, SES characteristics, and indicators of TB severity (bacillary density in sputum and Karnofsky performance score). Similar comparisons were carried out with body composition variables from bioelectrical impedance analysis and albumin concentrations, in a subsample of 731 subjects. -

Cancer Cachexia and Fatigue
CME Palliative care Cancer cachexia and mechanisms (Fig 1). The cachectic Other cachectic factors patient is analogous to an accelerating Cachexia can occur in the absence of car running out of petrol. The anorexia anorexia, suggesting that catabolic fatigue component of cancer cachexia reduces mediators produced by tumour or host fuel supply (by ca 300–500 kcal/day) cells are involved in the cancer cachexia whilst accelerated metabolic cycling Grant D Stewart BSc(Hons) MBChB MRCS(Ed), process.9 Experimental cachexia models drives hypermetabolism (by ca Surgical Research Fellow suggest pro-inflammatory cytokines, 100–200 kcal/day). There are also the Richard JE Skipworth BSc(Hons) MBChB such as tumour necrosis factor- , inter- direct catabolic effects of muscle proteol- α MRCS(Ed), Surgical Research Fellow leukin (IL)-6, IL-1 and interferon- , can ysis and lipolysis. These changes underlie γ Kenneth CH Fearon MBChB(Hons) MD all play a role. Activation of the neuro- a key paradox of cachexia: whilst meta- FRCS(Glas) FRCS(Ed) FRCS(Eng), Professor of endocrine stress response is also thought bolic rate may be increased, overall (or Surgical Oncology to be important. Potential mediators total) energy expenditure is decreased Department of Clinical and Surgical Sciences include increased adrenergic activity, ele- due to a fall in physical activity.7 (Surgery), University of Edinburgh, Royal vated cortisol, low insulin and increased Infirmary, Edinburgh activity of the renin-angiotensin system.1 Anorexia With regard to tumour-specific Clin Med 2006;6:140–3 The anorexia component of cancer cachectic factors, proteolysis-inducing cachexia has both a neurohumoral mech- factor (PIF) is produced by tumours and anism due to disturbance of the central excreted in the urine of patients with Background physiological mechanisms controlling cancer cachexia. -
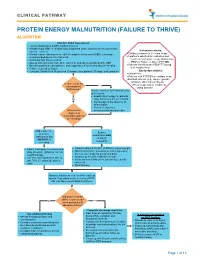
Protein Energy Malnutrition (Failure to Thrive) Algorithm
CLINICAL PATHWAY PROTEIN ENERGY MALNUTRITION (FAILURE TO THRIVE) ALGORITHM Conduct Initial Assessment • History and physical (H&P), nutrition focused • Weight height, BMI, % of ideal body weight and exam: assess severity (symmetric edema = severe) Inclusion criteria: • • Consider basic labs based on H&P; A complete blood count (CBC) is strongly Children newborn to 21 years of age • recommended due to risk of anemia Inpatients admitted for evaluation and • Additional labs based on H&P treatment of Protein Energy Malnutrition • Assess micronutrients: iron, zinc, vitamin D, and others as indicated by H&P (PEM) or Failure to thrive (FTT) OR • • Baseline potassium, phosphorus, and magnesium if concerned about re-feeding Patients identified with PEM/FTT during • Calorie count up to 3 days their hospital stay. • Consults: Social Work, Registered Dietician, Occupational Therapy, and Lactation Exclusion criteria: • Outpatients • Patients with FTT/PEM secondary to an identified concern (e.g., cancer, genetic condition, other chronic illness). Is there a risk for •Pts w/ suspected or confirmed micronutrient Yes eating disorder deficiencies? Initiate treatment for micronutrients deficiencies: • Empiric zinc therapy for patients No older than 6 months for 1 month • Iron therapy in the absence of inflammation • Vitamin D and other What are the micronutrients based on labs degrees of malnutrition and risk of refeeding? Mild, moderate, Severe or severe malnutrition AND malnutrition but at risk of NO RISK of refeeding refeeding • • Initiate feeding per recommended Initiate feeding at 30-50% of RDA for current weight • daily allowance (RDA) for current Monitor potassium, phosphorus, and magnesium weight and age once to twice a day for a total of 4 days • • Use PO route if patient is able to Advance by 10-20% if labs are normal • take 70% of estimated calories If labs abnormal hold off on advancing feed until orally corrected • Start thiamine Advance calories to meet level for catch up growth. -

Mechanism of Hypokalemia in Magnesium Deficiency
SCIENCE IN RENAL MEDICINE www.jasn.org Mechanism of Hypokalemia in Magnesium Deficiency Chou-Long Huang*† and Elizabeth Kuo* *Department of Medicine, †Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center, Dallas, Texas ABSTRACT deficiency is likely associated with en- ϩ Magnesium deficiency is frequently associated with hypokalemia. Concomitant hanced renal K excretion. To support magnesium deficiency aggravates hypokalemia and renders it refractory to treat- this idea, Baehler et al.5 showed that ad- ment by potassium. Herein is reviewed literature suggesting that magnesium ministration of magnesium decreases ϩ deficiency exacerbates potassium wasting by increasing distal potassium secre- urinary K excretion and increases se- ϩ tion. A decrease in intracellular magnesium, caused by magnesium deficiency, rum K levels in a patient with Bartter releases the magnesium-mediated inhibition of ROMK channels and increases disease with combined hypomagnesemia potassium secretion. Magnesium deficiency alone, however, does not necessarily and hypokalemia. Similarly, magnesium ϩ cause hypokalemia. An increase in distal sodium delivery or elevated aldosterone replacement alone (without K ) in- ϩ levels may be required for exacerbating potassium wasting in magnesium creases serum K levels in individuals deficiency. who have hypokalemia and hypomag- nesemia and receive thiazide treatment.6 J Am Soc Nephrol 18: 2649–2652, 2007. doi: 10.1681/ASN.2007070792 Magnesium administration decreased urinary Kϩ excretion in these individuals (Dr. Charles Pak, personal communica- Hypokalemia is among the most fre- cin B, cisplatin, etc. Concomitant magne- tion, UT Southwestern Medical Center quently encountered fluid and electro- sium deficiency has long been appreci- at Dallas, July 13, 2007).