Annual Report 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

50 KW Offer Solar Copy 2
21st Century Enviro Engineers Pvt.Ltd. Plot No. 120(10 Marla), COMPANY Industrial Area Phase II Chandigarh - 160002, India PROFILE www.21stcenturyenviro.com INTRODUCTION 21st CENTURY ENVIRO ENGINEERS PVT. LTD. is a Company dealing in the field of Environmental Engineering related activities. We are Registered Environmental Consultants of Pollution Control Board to Supply ETP’s, STP’s, APCD’s, Incinerators, Water Treatment Plants, Reverse Osmosis, Evaporators, Solid Waste Management and Rain Water Harvesting and to carry out EIA Studies . The Directors of the company are young Technocrats having versatile experience in this field. We have a back up of highly qualified and experienced technical team having versatile experience in Designing, Erection, Commissioning and Operation of different types of Effluent Treatment Plants. Our Managing Director himself is a Chemical Engineer with almost 25 Years Experience in this line and Technical Director is PhD. in Environmental Science with almost 30 Years working experience on various types of effluent treatment Technologies. Our team comprises of almost 150 people from different backgrounds e.g. Chemical, Environmental, Mechanical, Instrumentation, Civil, Accounts, Purchase, Marketing etc. We have our Marketing/ Design Office in Chandigarh and regional offices in Dhaka, Mumbai, New Delhi, Sikkim etc. and have our own testing Laboratory for Effluent/Water Testing and to study treatability of effluent on pilot scale. We have our own full-fledged manufacturing unit of Pollution Control Equipments in Village Kunjhal Baddi (HP). We also undertake annual operation and maintenance contracts and liasining services for the systems supplied by us. We are also Registered with CREST (Chandigarh Renewable Energy & Technology Promotion Society) and SECI (Solar Energy Corporation of India) for supplying Online/Offline Solar systems in Chandigarh, Himachal Pradesh, Uttarakhand, J&K, Punjab, Haryana, Delhi, Bihar etc. -

Glenmark Pharmaceuticals (GLEPHA)
Glenmark Pharmaceuticals (GLEPHA) CMP: | 483 Target: | 560 (16%) Target Period: 12 months BUY August 17, 2020 Subdued topline; margin expansion to the fore… Revenues stayed flattish growing just 0.9% YoY to | 2345 crore due to subdued growth of 1.6% in US to | 743 crore, 3.7% growth in domestic sales to | 780 crore. Europe business grew 12.8% YoY to | 274 crore. RoW markets de-grew 18.1% YoY to 212 crore. EBITDA margins improved 567 bps YoY to 20.4% mainly due to lower other expenditure. EBITDA grew Particulars 39.8% YoY to | 478 crore. Adjusted PAT grew 106.9% YoY to | 226 crore. P articular Am ount Delta vis-à-vis EBITDA was due to higher other income. Exceptional items in Market Capitalisation | 13615 crore Q1FY21 were | 28 crore on gain arising from sale of Vwash brand to HUL. Debt (FY20) | 4868 crore Result Update Result Cash & equivalent (FY20) | 1111 crore US growth dependent on new launches E V | 17371 crore 52 week H/L (|) 573/168 US generics comprise ~30% of total revenues. So far, the company has E quity capital | 28.2 crore Face value | 1 received approval for 164 ANDAs while another 44 are pending approval, of which 24 are Para IV applications. However, Glenmark’s derma portfolio is Key Highlights facing stiff pricing pressure in the US. Going ahead, traction from the newly commissioned US based Monroe facility will be the key determinant besides Q1FY21 revenues remained flattish sustained product launches. We expect the US to grow at 5.2% CAGR in due to subdued US growth amid FY20-22E to | 3479 crore on the back of new launches. -

United Spirits Limited Corporate Identity Number: L01551KA1999PLC024991 Registered Office: ‘UB Tower’, #24, Vittal Mallya Road, Bangalore - 560 001
United Spirits Limited Corporate Identity Number: L01551KA1999PLC024991 Registered Office: ‘UB Tower’, #24, Vittal Mallya Road, Bangalore - 560 001. Tel: +91 80 3985 6500; Fax: +91 80 3985 6862; www.unitedspirits.in, Email: [email protected] NOTICE NOTICE IS HEREBY GIVEN OF THE FIFTEENTH ANNUAL GENERAL MEETING (“AGM”) of United Spirits Limited (the “Company”) to be held at Level 1, UB Tower, #24, Vittal Mallya Road, Bangalore 560 001 on Tuesday, September 30, 2014 at 2.30 p.m. for the following purposes: ORDINARY BUSINESS 1. To receive, consider and adopt the Audited Statement of Profit and Loss for the financial year ended March 31, 2014, the Balance Sheet as at that date and the Reports of the Directors and Auditors thereon. 2. To appoint a Director in place of Dr. Vijay Mallya (DIN: 00122890), who retires by rotation and being eligible, offers himself for re-appointment. 3. To consider and if thought fit, to pass with or without modification(s), the following resolution as an Ordinary Resolution: RESOLVED that the vacancy in the Board of Directors of the Company arising out of the retirement of Mr. Gilbert Ghostine (DIN: 06555302) who retires by rotation at this AGM and has not offered himself for re-appointment, not be filled up as of the current date. 4. To appoint Statutory Auditors and to fix their remuneration. To consider and if thought fit, to pass with or without modification(s), the following resolution as an Ordinary Resolution: RESOLVED that pursuant to the provisions of Section 139 of the Companies Act, 2013 and the rules made thereunder, and pursuant to the recommendation of the Audit Committee of the Board of Directors, M/s. -

Hy Sun Missed the Pharma Rally: the Answer Is Hidden in a Bet Many Failed — Speciality Drugs - the Economic Times
12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets News Industry RISE Politics Wealth MF Tech Jobs Opinion NRI Panache ET NOW More Aayush English Edition | E-Paper Tech Consumer Markets Corporate Governance Telecom + OTT Auto + Aviation Pharma Fintech + BFSI Economy Infra Environment Energy Business News › Prime › Pharma › Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Getty Images MARKETS hy Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs Dilip Shanghvi, founder and managing director, Sun Pharmaceuticals Synopsis Sun Pharma is the only Indian company to have made some inroads into speciality drugs. What worries investors is high investments and uncertainties over ramp up in revenue. The stock can still see upside because of its current valuations, strong India business, and any positive surprises in US generic business. But it is crucial that its speciality bet pays off. BACK TO TOP https://economictimes.indiatimes.com/prime/pharma-and-healthcare/why-sun-missed-the-pharma-rally-the-answer-is-hidden-in-a-bet-many-failed-spe… 1/11 12/3/2020 Sun Pharma: Why Sun missed the pharma rally: the answer is hidden in a bet many failed — speciality drugs - The Economic Times Home ETPrime Markets NeCwasllI nitd uas trbyleRssISiEngP oilniti cdsisWgeuailthse fMoFr tTheceh InJodbisanOpinion NRI Panache ET NOW More pharmaceutical industry. The stocks of pharma companies have been on a tear since the beginning of the Covid-19 crisis. -

DIA India ICH Day 2019 Enhancing Clinical Trial Outcomes with ICH 30Th August, 2019 | Taj Lands End | Mumbai, India
DIA India ICH Day 2019 Enhancing Clinical Trial Outcomes with ICH 30th August, 2019 | Taj Lands End | Mumbai, India PROGRAMME COMMITTEE Today, the Indian pharmaceutical industry supplies approximately 20% of the world’s generic drugs and has the largest number of FDA-approved manufacturing facilities C. Michelle Limoli outside of the US. This industry has evolved during the past decade into innovative drug Senior International development, including new chemical and biological entities, biosimilars, and innovative Health Science Advisor differentiated products. At the same time, as healthcare costs continue to rise across the US FDA world, so does the global need for affordable, innovative healthcare. Against this backdrop, DIA India aims to create an initiative that will address the clinical and regulatory aspects Ramesh Jagannathan of drug development, through a comprehensive single day workshop on the latest ICH Associate Vice President guidelines and developments relevant for India. - R&D Biocon Ltd. Objective Anupama Ramkumar CEO and Principal The objective of this program is to provide a common platform for ICH trainers, regulatory Consultant authorities, academia, investigators, service providers, and the Indian biopharmaceutical Arkus Research Pvt. Ltd. industry, to deliberate upon and understand ICH guidelines and the most recent updates, impacting the drug development spectrum including key clinical, regulatory and quality Seema Pai aspects. Director - India Cluster Global Site & Study Take a look at our key topics for the event -
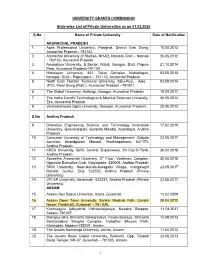
UNIVERSITY GRANTS COMMISSION State-Wise List of Private
UNIVERSITY GRANTS COMMISSION State-wise List of Private Universities as on 01.02.2020 S.No Name of Private University Date of Notification ARUNACHAL PRADESH 1. Apex Professional University, Pasighat, District East Siang, 10.05.2013 Arunachal Pradesh - 791102. 2. Arunachal University of Studies, NH-52, Namsai, Distt – Namsai 26.05.2012 - 792103, Arunachal Pradesh. 3. Arunodaya University, E-Sector, Nirjuli, Itanagar, Distt. Papum 21.10.2014 Pare, Arunachal Pradesh-791109 4. Himalayan University, 401, Takar Complex, Naharlagun, 03.05.2013 Itanagar, Distt – Papumpare – 791110, Arunachal Pradesh. 5. North East Frontier Technical University, Sibu-Puyi, Aalo 03.09.2014 (PO), West Siang (Distt.), Arunachal Pradesh –791001. 6. The Global University, Hollongi, Itanagar, Arunachal Pradesh. 18.09.2017 7. The Indira Gandhi Technological & Medical Sciences University, 26.05.2012 Ziro, Arunachal Pradesh. 8. Venkateshwara Open University, Itanagar, Arunachal Pradesh. 20.06.2012 S.No Andhra Pradesh 9. Bharatiya Engineering Science and Technology Innovation 17.02.2019 University, Gownivaripalli, Gorantla Mandal, Anantapur, Andhra Pradesh 10. Centurian University of Technology and Management, Gidijala 23.05.2017 Junction, Anandpuram Mandal, Visakhapatnam- 531173, Andhra Pradesh. 11. KREA University, 5655, Central, Expressway, Sri City-517646, 30.04.2018 Andhra Pradesh 12. Saveetha Amaravati University, 3rd Floor, Vaishnavi Complex, 30.04.2018 Opposite Executive Club, Vijayawada- 520008, Andhra Pradesh 13. SRM University, Neerukonda-Kuragallu Village, mangalagiri 23.05.2017 Mandal, Guntur, Dist- 522502, Andhra Pradesh (Private University) 14. VIT-AP University, Amaravati- 522237, Andhra Pradesh (Private 23.05.2017 University) ASSAM 15. Assam Don Bosco University, Azara, Guwahati 12.02.2009 16. Assam Down Town University, Sankar Madhab Path, Gandhi 29.04.2010 Nagar, Panikhaiti, Guwahati – 781 036. -

Company Reliance Industries Limited Tata Consultancy Services
Top 1000 Private Sector Companies (Rank-wise List) Company Reliance Industries Limited Tata Consultancy Services (TCS) Infosys Technologies Ltd Wipro Limited Bharti Tele-Ventures Limited ITC Limited Hindustan Lever Limited ICICI Bank Limited Housing Development Finance Corp. Ltd. TATA Steel Limited Ranbaxy Laboratories Limited HDFC Bank Ltd Tata Motors Limited Larsen & Toubro Limited (L&T) Satyam Computer Services Ltd. Maruti Udyog Limited Bajaj Auto Ltd. HCL Technologies Ltd. Hero Honda Motors Limited Hindalco Industries Ltd Reliance Energy Limited Grasim Industries Limited Jet Airways (India) Ltd. Sun Pharmaceuticals Industries Ltd Cipla Ltd. Gujarat Ambuja Cements Ltd. Videsh Sanchar Nigam Limited The Tata Power Company Limited Sterlite Industries (India) Ltd. Associated Cement Companies Ltd. Nestlé India Ltd. Hindustan Zinc Limited GlaxoSmithKline Pharmaceuticals Limited Siemens India Ltd. Motor Industries Company Limited Mahindra & Mahindra Limited UTI Bank Ltd. Zee Telefilms Limited Bharat Forge Limited ABB Limited i-Flex Solutions Ltd. Dr. Reddy's Laboratories Ltd. Nicholas Piramal India Limited Kotak Mahindra Bank Limited Reliance Capital Ltd. Ultra Tech Cement Ltd. Patni Computer Systems Ltd. Wockhardt Limited Indian Petrochemicals Corporation Limited Biocon India Limited Essar Oil Limited. Asian Paints Ltd. Dabur India Limited Jaiprakash Associates Limited JSW Steel Limited Tata Chemicals Limited Tata Tea Limited Tata Teleservices (Maharashtra) Limited The Indian Hotels Co. Ltd. Glenmark Pharmaceuticals Limited NIRMA Limited Jindal Steel & Power Ltd HCL Infosystems Ltd. Cadila Healthcare Limited Colgate-Palmolive (India) Limited The Great Eastern Shipping Company Limited Aventis Pharma India Ltd Ashok Leyland Limited Pantaloon Retail (India) Limited Indian Rayon And Industries Limited Financial Technologies (India) Ltd United Phosphorus Limited Matrix Laboratories Limited Sesa Goa Limited Lupin Ltd Cummins India Limited Crompton Greaves Limited. -

Final Placement Report 2020-21
FINAL PLACEMENT REPORT POST GRADUATE ProgrAM IN MANAGEMENT (PGP) 2019-21 12th BAtcH INDIAN INSTITUTE OF MANAGEMENT SHILLONG FOREWORD The Placement Committee of IIM Shillong takes immense pride in announcing the successful completion of the final placement season for the PGP batch of 2019-21. The stellar performance by the PGP participants stands testimony to the rapid progress made by IIM Shillong and has once again established IIM Shillong as the preferred choice for recruiters. We express our gratitude to our regular recruiters who have continuously believed in the mettle of our students and have furthered their association with us for another year to facilitate this mutually beneficial relationship. We are also grateful to our new recruiters, who have acknowledged the potential of our students and provided them with a plethora of opportunities. We appreciate the contribution of the entire IIM Shillong fraternity for their sustained assistance in the placement process. With continued support from students, recruiters, faculty, alumni, staff and management, we hope to carry this momentum onto the next academic year. Prof. Rohit Joshi Chairperson Placement Committee IIM Shillong BATCH PROFILE OF 2019-21 Education Background 9% 9% 9% 57% 198 16% Number 61% 39% of Students Male Female Engineering Arts Commerce & Finance Others Science Work Experience by Months Domain wise Work Experience Freshers 27% Information Technology & Analytics 47% 01-12 months 23% Consulting & Strategy 13% 19% Finance 23% 13-24 months 18% Sales & Marketing 11% 25-36 -

List of Registered Colonies Approved Under Hp Apartment
LIST OF REGISTERED COLONIES APPROVED UNDER H.P. APARTMENT AND PROPERTY REGULATION ACT, 2005 Sr. Name and Address of the Location of Project Licence No. & dated No Promoter . 1 2 3 4 Year-2005 (02) 1 Sh. Amarnath Aggarwal Village Dakru Pargana-Nalagarh, HIMUDA/LIC-1/2005 M/s Amar Nath Aggarwal Tehsil Nalagarh, District Solan, dated 5.10.2005. Builders Pvt. Ltd. Office 407, H.P. Motor Market, Mani Majra Chandigarh 2. Sh. Harish Aggarwal, M/s Mauza Katha Pargana, HIMUDA/LIC-2/2005 Mount View Group Housing Co. Dharampur( Baddi), Teshil dated 5.12.2005 Plot No.176, HPSIDC, Industrial Nalagarh, District Solan, H.P. Area, Baddi, District Solan, H.P. Year-2006 (11) 3. Sh. Lalit Jindal, M/s Hill View Village Bhatouli Kalan, Pargana- HIMUDA/LIC-3/2006 Infrastructure Pvt. Ltd-2048, Dharampur, Tehsil Nalagarh, dated 5.5.2006. Sector-15-C, Chandigarh, U.T. District Solan, H.P. 4. Sh. Amarnath Aggarwal, M/s Village Dakru Pargana-Nalagarh, HIMUDA/LIC-4/2006 Amar Nath Aggarwal Builders Tehsil Nalagarh, District Solan, dated 12.5.2006. Pvt. Ltd. Regd. Office, 407 H.P. Motor Market Manimajra Chandigarh and Central Office SCO-10-11, Sector, Panchkulla, Haryana 5. Sh. Ashwin Johar, M/s MDC Village Bhatouli Kalan, Pargana, HIMUDA/LIC-5/2006 Estates Pvt. Ltd. 319, Sector-21- Dharampur, Tehsil Nalagarh, dated 17.6.2006 A, Chandigarh U.T. District Solan, H.P. 6. Sh. Daleep Moudgil, M/s Omax Village Billanwali Gujran HIMUDA/LIC-6/2006 Construction Ltd.,7-Local Pargana-Dharampur, Tehsil dated 4.7.2006 Shopping Centre, Omax House, Nalagarh, District Solan, H.P. -

Application for Appointment on the Post of Assistant Professor
I Block-202, Gillco Towers, Gillco Valley, Kharar,Sector 127, Mohali, Punjab E-mail: [email protected] Mobile: 91-8628885639, 91-8894987978 Dr. GEORGE THOMAS OBJECTIVE My goal is to be associated with an institution that can utilize my experience and skills; hereafter augmenting both its productivity and position that will eventually amalgamate into my growth and expansion. PROFESSIONAL ASSOCIATION: Life member of All India Management Association (AIMA), Membership No. LM- 200811196, since 1st April, 2008 Former Joint Secretary of Gwalior Management Association, January 2013-14 Counselor for M.B.A. program for Gwalior Chapter in Indira Gandhi National Open University (IGNOU), ID No. 04136 Editor/ Member Editorial Board Editor-in-Chief of ‘The Catalyst Journal of Management’, a refereed bi-annual Journal of School of Management Studies, Baddi University of Emerging Sciences and Technology, Baddi, Himachal Pradesh ISSN: 2455-7929 Editor of the ‘International Journal of Business Innovation’, a refereed bi-annual international journal of School of Management, Maharaja Agrasen University, Baddi, Himachal Pradesh, ISSN:2394-8086 Managing Editor of ‘Agrani Samkalp’, a bi-annual newsletter of Maharaja Agrasen University, Baddi, Himachal Pradesh, Reg. No. DELBIL/2004/13585 Member of the Editorial Board of ‘Tourism Dimensions’, a refereed International Journal of Tourism, Maharaja Agrasen University, Baddi, Himachal Pradesh, ISSN: 2349-7394 Total Work Experience: 22 years since 1994 17 years of Teaching Experience, including 8 -
View Annual Report
CONTENTS CHAIRMAN’S LETTER DEAR SHAREHOLDERS FY2012 has been a good year for your Company. The key financial results were: ¥ Consolidated revenues increased by 30% to Rs. 96.7 billion in FY2012. ¥ Earnings before interest, taxes, depreciation and amortization (EBITDA)1 rose by 55% to Rs. 25.4 billion. ¥ Profit after Tax (PAT)2 grew by 45% to Rs. 15.3 billion. ¥ Diluted Earnings per Share (EPS) increased from Rs. 64.9 in FY2011 to Rs. 83.8 in FY2012. I am particularly delighted by four developments. First, your Company succeeded in yet another blockbuster generic launch in the USA under 180- days marketing exclusivity. Dr. Reddy’s launched olanzapine 20 mg tablets, the generic version of the brand Zyprexa®. Olanzapine is used to treat schizophrenia and bipolar disorder. This product has added around USD 100 million to your Company’s revenues for FY2012. Second, the biosimilars business continues along its impressive growth path. In my letter to you last year, I had discussed the critical importance of developing biosimilars in the years to come. I am happy to note that your Company’s global biosimilars business grew by 45% over last year and recorded sales of USD 26 million. Today, the biosimilars portfolio of Dr. Reddy’s constitutes (i) filgrastim, (ii) peg-filgrastim, (iii) rituximab and (iv) darbepoetin alfa, which have commercial presence in 13 countries among emerging markets. These are helping to treat patients suffering from cancer — and at prices that are significantly more affordable than the corresponding innovator drugs. Soon, I expect to see Dr. Reddy’s biosimilars entering developed markets. -

Dated: A8)10)1, to ,,An.Nodal Officer-Cum-APCCF(FCA) "./ Office of Pr
No.DlC/swcA/BD/1.A./Dabhota/- 22 3q Office of the Dy.Director of lndustries Single Window Clearance Agency, Baddi Distt.Solan H.P. Dated: A8)10)1, To ,,An.Nodal officer-cum-APCCF(FCA) "./ Office of Pr. CCF, H.P., Shimla-1. Subject:- Diversion of 38.7 ha. Of forest land in favor of Deputy Director of Industries for setting up of New lndustrialArea at village Dabhota, Tehsil Nalagarh, Distt. Solan, H.P. Sir, ln reference to your letter No. F|.48-281612014(FCA), dated 04.10.2014, on the subject quoted above vide which following shortcomings were shown and the same have been completed and enclosed herewith for taking further action in the matter please. S.No. Shorlcominq Remarks 1. ln the justification for locating the project in forest area, no Proper justification justification for locating this project on forest land has been report enclosed. given, only the justification for the project has been given. The requirement of this check list is why the project is to be located over the forest land and not on the other Pvt/non- forest land. Thus, proper justification for locating the project on forest land is required to be uploaded. 2. Detail of alternates examined to reduce the requirement of Detail of alternates forest land need to be provided. examined enclosed. 3. Although the component wise detail has been uploaded, but Dimensions of the dimensions of the project components are also required to project components uoloaded. enclosed. 4. Underlaking of the user agency to bear the cost of CA has not Undertaking of the been uploaded.