The Early Evolution of the Tetrapod Humerus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

How Plesiosaurs Swam: New Insights Into Their Underwater Flight Using “Ava”, a Virtual Pliosaur
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 9 October 2019 doi:10.20944/preprints201910.0094.v1 How Plesiosaurs Swam: New Insights into Their Underwater Flight Using “Ava”, a Virtual Pliosaur Max Hawthorne1,*, Mark A. S. McMenamin 2, Paul de la Salle3 1Far From The Tree Press, LLC, 4657 York Rd., #952, Buckingham, PA, 18912, United States 2Department of Geology and Geography, Mount Holyoke College, South Hadley, Massachusetts, United States 3Swindon, England *Correspondence: [email protected]; Tel.: 267-337-7545 Abstract Analysis of plesiosaur swim dynamics by means Further study attempted to justify the use of all four flippers of a digital 3D armature (wireframe “skeleton”) of a simultaneously via the use of paddle-generated vortices, pliosauromorph (“Ava”) demonstrates that: 1, plesiosaurs which require specific timing to achieve optimal additional used all four flippers for primary propulsion; 2, plesiosaurs thrust. These attempts have largely relied on anatomical utilized all four flippers simultaneously; 3, respective pairs studies of strata-compressed plesiosaur skeletons, and/or of flippers of Plesiosauridae, front and rear, traveled through preconceived notions as pertains to the paddles’ inherent distinctive, separate planes of motion, and; 4, the ability to ranges of motion [8, 10-12]. What has not been considered utilize all four paddles simultaneously allowed these largely are the opposing angles of the pectoral and pelvic girdles, predatory marine reptiles to achieve a significant increase in which strongly indicate varied-yet-complementing relations acceleration and speed, which, in turn, contributed to their between the front and rear sets of paddles, both in repose and sustained dominance during the Mesozoic. -
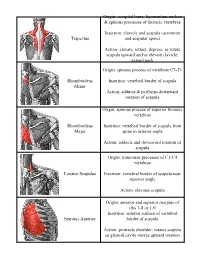
Trapezius Origin: Occipital Bone, Ligamentum Nuchae & Spinous Processes of Thoracic Vertebrae Insertion: Clavicle and Scapul
Origin: occipital bone, ligamentum nuchae & spinous processes of thoracic vertebrae Insertion: clavicle and scapula (acromion Trapezius and scapular spine) Action: elevate, retract, depress, or rotate scapula upward and/or elevate clavicle; extend neck Origin: spinous process of vertebrae C7-T1 Rhomboideus Insertion: vertebral border of scapula Minor Action: adducts & performs downward rotation of scapula Origin: spinous process of superior thoracic vertebrae Rhomboideus Insertion: vertebral border of scapula from Major spine to inferior angle Action: adducts and downward rotation of scapula Origin: transverse precesses of C1-C4 vertebrae Levator Scapulae Insertion: vertebral border of scapula near superior angle Action: elevates scapula Origin: anterior and superior margins of ribs 1-8 or 1-9 Insertion: anterior surface of vertebral Serratus Anterior border of scapula Action: protracts shoulder: rotates scapula so glenoid cavity moves upward rotation Origin: anterior surfaces and superior margins of ribs 3-5 Insertion: coracoid process of scapula Pectoralis Minor Action: depresses & protracts shoulder, rotates scapula (glenoid cavity rotates downward), elevates ribs Origin: supraspinous fossa of scapula Supraspinatus Insertion: greater tuberacle of humerus Action: abduction at the shoulder Origin: infraspinous fossa of scapula Infraspinatus Insertion: greater tubercle of humerus Action: lateral rotation at shoulder Origin: clavicle and scapula (acromion and adjacent scapular spine) Insertion: deltoid tuberosity of humerus Deltoid Action: -

Coracoid Process Anatomy: a Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B
Quantitative Assessment of the Coracoacromial and the Coracoclavicular Ligaments With 3-Dimensional Mapping of the Coracoid Process Anatomy: A Cadaveric Study of Surgically Relevant Structures Jorge Chahla, M.D., Ph.D., Daniel Cole Marchetti, B.A., Gilbert Moatshe, M.D., Márcio B. Ferrari, M.D., George Sanchez, B.S., Alex W. Brady, M.Sc., Jonas Pogorzelski, M.D., M.H.B.A., George F. Lebus, M.D., Peter J. Millett, M.D., M.Sc., Robert F. LaPrade, M.D., Ph.D., and CAPT Matthew T. Provencher, M.D., M.C., U.S.N.R. Purpose: To perform a quantitative anatomic evaluation of the (1) coracoid process, specifically the attachment sites of the conjoint tendon, the pectoralis minor, the coracoacromial ligament (CAL), and the coracoclavicular (CC) ligaments in relation to pertinent osseous and soft tissue landmarks; (2) CC ligaments’ attachments on the clavicle; and (3) CAL attachment on the acromion in relation to surgically relevant anatomic landmarks to assist in planning of the Latarjet procedure, acromioclavicular (AC) joint reconstructions, and CAL resection distances avoiding iatrogenic injury to sur- rounding structures. Methods: Ten nonpaired fresh-frozen human cadaveric shoulders (mean age 52 years, range 33- 64 years) were included in this study. A 3-dimensional coordinate measuring device was used to quantify the location of pertinent bony landmarks and soft tissue attachment areas. The ligament and tendon attachment perimeters and center points on the coracoid, clavicle, and acromion were identified and subsequently dissected off the bone. Coordinates of points along the perimeters of attachment sites were used to calculate areas, whereas coordinates of center points were used to determine distances between surgically relevant attachment sites and pertinent bony landmarks. -

Dolphin P-K Teacher's Guide
Dolphin P-K Teacher’s Guide Table of Contents ii Goal and Objectives iii Message to Our Teacher Partners 1 Dolphin Overview 3 Dolphin Activities 23 Dolphin Discovery Dramatic Play 7 Which Animals Live with 25 Dolphins? Picture This: Dolphin Mosaic 9 Pod Count 27 Dolphins on the Move 13 How Do They Measure Up? 31 Where Do I Live? Food Search 15 dorsal dorsal 35 Dolphin or fin peduncle Other Sea Creature? median blowhole notch posterior Build a anterior fluke17s melon 37 pectoral Dolphin Recycling flipper eye rostrum ear Can Make a bottlenose dolphin Difference! 19 ventral lengDolphinth = 10-14 feet / 3-4.2 meters Hokeypokey 41 d Vocabularyi p h o l n Goal and Message to Our Objectives Teacher Partners At l a n t i s , Paradise Island, strives to inspire students to learn Goal: Students will develop an understanding of what a more about the ocean that surrounds dolphin is and where it lives. them in The Bahamas. Through interactive, interdisciplinary activities in the classroom and at Atlantis, we endeavor to help students develop an understanding of the marine world along with Upon the completion of the Dolphin W e a r e the desire to conserve it and its wildlife. Dolphin Cay Objectives: provides students with a thrilling and inspirational program, students will be able to: a resource for you. Atlantis, Paradise Island, offers opportunity to learn about dolphins and their undersea a variety of education programs on world as well as ways they can help conserve them. themes such as dolphins, coral reefs, sharks, Through students’ visit to Atlantis, we hope to Determine which animals live in the ocean like dolphins. -

The Devonian Tetrapod Acanthostega Gunnari Jarvik: Postcranial Anatomy, Basal Tetrapod Interrelationships and Patterns of Skeletal Evolution M
Transactions of the Royal Society of Edinburgh: Earth Sciences, 87, 363-421, 1996 The Devonian tetrapod Acanthostega gunnari Jarvik: postcranial anatomy, basal tetrapod interrelationships and patterns of skeletal evolution M. I. Coates ABSTRACT: The postcranial skeleton of Acanthostega gunnari from the Famennian of East Greenland displays a unique, transitional, mixture of features conventionally associated with fish- and tetrapod-like morphologies. The rhachitomous vertebral column has a primitive, barely differentiated atlas-axis complex, encloses an unconstricted notochordal canal, and the weakly ossified neural arches have poorly developed zygapophyses. More derived axial skeletal features include caudal vertebral proliferation and, transiently, neural radials supporting unbranched and unsegmented lepidotrichia. Sacral and post-sacral ribs reiterate uncinate cervical and anterior thoracic rib morphologies: a simple distal flange supplies a broad surface for iliac attachment. The octodactylous forelimb and hindlimb each articulate with an unsutured, foraminate endoskeletal girdle. A broad-bladed femoral shaft with extreme anterior torsion and associated flattened epipodials indicates a paddle-like hindlimb function. Phylogenetic analysis places Acanthostega as the sister- group of Ichthyostega plus all more advanced tetrapods. Tulerpeton appears to be a basal stem- amniote plesion, tying the amphibian-amniote split to the uppermost Devonian. Caerorhachis may represent a more derived stem-amniote plesion. Postcranial evolutionary trends spanning the taxa traditionally associated with the fish-tetrapod transition are discussed in detail. Comparison between axial skeletons of primitive tetrapods suggests that plesiomorphic fish-like morphologies were re-patterned in a cranio-caudal direction with the emergence of tetrapod vertebral regionalisation. The evolution of digited limbs lags behind the initial enlargement of endoskeletal girdles, whereas digit evolution precedes the elaboration of complex carpal and tarsal articulations. -

Reconstructing Pectoral Appendicular Muscle Anatomy in Fossil Fish and Tetrapods Over the Fins-To-Limbs Transition
Biol. Rev. (2017), pp. 000–000. 1 doi: 10.1111/brv.12386 Reconstructing pectoral appendicular muscle anatomy in fossil fish and tetrapods over the fins-to-limbs transition Julia L. Molnar1,∗ , Rui Diogo2, John R. Hutchinson3 and Stephanie E. Pierce4 1Department of Anatomy, New York Institute of Technology College of Osteopathic Medicine, Northern Boulevard, Old Westbury, NY, U.S.A. 2Department of Anatomy, Howard University College of Medicine, 520 W St. NW, Numa Adams Building, Washington, DC 20059, U.S.A. 3Structure and Motion Lab, Royal Veterinary College, Hawkshead Lane, Hatfield, Hertfordshire AL9 7TA, UK 4Museum of Comparative Zoology and Department of Organismic and Evolutionary Biology, Harvard University, 26 Oxford Street, Cambridge, MA 02138, U.S.A. ABSTRACT The question of how tetrapod limbs evolved from fins is one of the great puzzles of evolutionary biology. While palaeontologists, developmental biologists, and geneticists have made great strides in explaining the origin and early evolution of limb skeletal structures, that of the muscles remains largely unknown. The main reason is the lack of consensus about appendicular muscle homology between the closest living relatives of early tetrapods: lobe-finned fish and crown tetrapods. In the light of a recent study of these homologies, we re-examined osteological correlates of muscle attachment in the pectoral girdle, humerus, radius, and ulna of early tetrapods and their close relatives. Twenty-nine extinct and six extant sarcopterygians were included in a meta-analysis using information from the literature and from original specimens, when possible. We analysed these osteological correlates using parsimony-based character optimization in order to reconstruct muscle anatomy in ancestral lobe-finned fish, tetrapodomorph fish, stem tetrapods, and crown tetrapods. -

Evolution of the Muscular System in Tetrapod Limbs Tatsuya Hirasawa1* and Shigeru Kuratani1,2
Hirasawa and Kuratani Zoological Letters (2018) 4:27 https://doi.org/10.1186/s40851-018-0110-2 REVIEW Open Access Evolution of the muscular system in tetrapod limbs Tatsuya Hirasawa1* and Shigeru Kuratani1,2 Abstract While skeletal evolution has been extensively studied, the evolution of limb muscles and brachial plexus has received less attention. In this review, we focus on the tempo and mode of evolution of forelimb muscles in the vertebrate history, and on the developmental mechanisms that have affected the evolution of their morphology. Tetrapod limb muscles develop from diffuse migrating cells derived from dermomyotomes, and the limb-innervating nerves lose their segmental patterns to form the brachial plexus distally. Despite such seemingly disorganized developmental processes, limb muscle homology has been highly conserved in tetrapod evolution, with the apparent exception of the mammalian diaphragm. The limb mesenchyme of lateral plate mesoderm likely plays a pivotal role in the subdivision of the myogenic cell population into individual muscles through the formation of interstitial muscle connective tissues. Interactions with tendons and motoneuron axons are involved in the early and late phases of limb muscle morphogenesis, respectively. The mechanism underlying the recurrent generation of limb muscle homology likely resides in these developmental processes, which should be studied from an evolutionary perspective in the future. Keywords: Development, Evolution, Homology, Fossils, Regeneration, Tetrapods Background other morphological characters that may change during The fossil record reveals that the evolutionary rate of growth. Skeletal muscles thus exhibit clear advantages vertebrate morphology has been variable, and morpho- for the integration of paleontology and evolutionary logical deviations and alterations have taken place unevenly developmental biology. -

Late Cretaceous) of Morocco : Palaeobiological and Behavioral Implications Remi Allemand
Endocranial microtomographic study of marine reptiles (Plesiosauria and Mosasauroidea) from the Turonian (Late Cretaceous) of Morocco : palaeobiological and behavioral implications Remi Allemand To cite this version: Remi Allemand. Endocranial microtomographic study of marine reptiles (Plesiosauria and Mosasauroidea) from the Turonian (Late Cretaceous) of Morocco : palaeobiological and behavioral implications. Paleontology. Museum national d’histoire naturelle - MNHN PARIS, 2017. English. NNT : 2017MNHN0015. tel-02375321 HAL Id: tel-02375321 https://tel.archives-ouvertes.fr/tel-02375321 Submitted on 22 Nov 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. MUSEUM NATIONAL D’HISTOIRE NATURELLE Ecole Doctorale Sciences de la Nature et de l’Homme – ED 227 Année 2017 N° attribué par la bibliothèque |_|_|_|_|_|_|_|_|_|_|_|_| THESE Pour obtenir le grade de DOCTEUR DU MUSEUM NATIONAL D’HISTOIRE NATURELLE Spécialité : Paléontologie Présentée et soutenue publiquement par Rémi ALLEMAND Le 21 novembre 2017 Etude microtomographique de l’endocrâne de reptiles marins (Plesiosauria et Mosasauroidea) du Turonien (Crétacé supérieur) du Maroc : implications paléobiologiques et comportementales Sous la direction de : Mme BARDET Nathalie, Directrice de Recherche CNRS et les co-directions de : Mme VINCENT Peggy, Chargée de Recherche CNRS et Mme HOUSSAYE Alexandra, Chargée de Recherche CNRS Composition du jury : M. -

Breathing and Locomotion in Birds
Breathing and locomotion in birds. A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Life Sciences. 2010 Peter George Tickle Contents Abstract 4 Declaration 5 Copyright Statement 6 Author Information 7 Acknowledgements 9 Organisation of this PhD thesis 10 Chapter 1 General Introduction 13 1. Introduction 14 1.1 The Avian Respiratory System 14 1.1.1 Structure of the lung and air sacs 16 1.1.2 Airflow in the avian respiratory system 21 1.1.3 The avian aspiration pump 25 1.2 The uncinate processes in birds 29 1.2.1 Uncinate process morphology and biomechanics 32 1.3 Constraints on breathing in birds 33 1.3.1 Development 33 1.3.2 Locomotion 35 1.3.2.1 The appendicular skeleton 35 1.3.2.2 Overcoming the trade-off between breathing 36 and locomotion 1.3.2.3 Energetics of locomotion in birds 38 1.4 Evolution of the ventilatory pump in birds 41 1.5 Overview and Thesis Aims 42 2 Chapter 2 Functional significance of the uncinate processes in birds. 44 Chapter 3 Ontogenetic development of the uncinate processes in the 45 domestic turkey (Meleagris gallopavo). Chapter 4 Uncinate process length in birds scales with resting metabolic rate. 46 Chapter 5 Load carrying during locomotion in the barnacle goose (Branta 47 leucopsis): The effect of load placement and size. Chapter 6 A continuum in ventilatory mechanics from early theropods to 48 extant birds. Chapter 7 General Discussion 49 References 64 3 Abstract of a thesis by Peter George Tickle submitted to the University of Manchester for the degree of PhD in the Faculty of Life Sciences and entitled ‘Breathing and Locomotion in Birds’. -

Some Notes on the Diverse Brachiosaurid Sauropods of the Late Jurassic of North America, Europe and Africa
Some Notes on the Diverse Brachiosaurid Sauropods of the Late Jurassic of North America, Europe and Africa GREGORY S. PAUL 3109 N. Calvert St. Baltimore, Maryland 21218 USA [email protected] June 2012 Abstract: The unusual placement of the parapophyses on elongotated peduncles further distinguishes Brachiosaurus from other brachiosaurid genera including Giraffatitan. Late Jurassic brachiosaurid taxonomy is probably more complex than has been realized, in part because of evolution over the considerable periods of times recorded in the Morrison and Tendaguru formations. The presence of reduced tails on late Jurassic brachiosaurids is confirmed. INTRODUCTION It was long assumed that the brachiosaurid material found in the Late Jurassic Morrison, Tendaguru, and Lourinha Formations from three continents belonged to the genus Brachiosaurus (as per Janensch 1950, 1961, Lapparent and Zbyszewski 1957, Jensen 1987) until Paul (1988) noted significant differences between the type species B. altithorax (Fig. 1A) from North America and the African B. brancai (Fig. 1B) indicated at least a subgeneric separation, and Taylor (2009) formally separated the sauropods, leaving much of the Tendaguru brachiosaurids material titled Giraffatitan brancai; the generic separation has been tentatively questioned (Chure et al. 2010). In addition European B. alatalaiensis has been retitled Lusotitan alatalaiensis (Antunes and Mateus 2003), and a dwarf brachiosaur from the European Mittlere Kimmeridge-Stufe deposits has been designated Europasaurus holgeri (Sander et al. 2006). Institutional Abbreviations: AMNH, American Museum of Natural History, New York; BYU, Brigham Young University, Provo; FMNH, Field Museum of Natural History, Chicago; HMN, Humboldt Museum fur Naturkunde, Berlin; NHMUK, Natural History Museum, London; USNM, United States Natural History Museum, Washington DC. -

Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K
Rehabilitation Guidelines for Latarjet/Coracoid Process Transfer Josef K. Eichinger, MD Shoulder instability may be caused from congenital deformity, recurrent overuse activity, and/or traumatic dislocation. Surgical stabilization of the glenohumeral joint is indicated after conservative treatment fails and recurrent instability/subluxation continues. A number of different surgical procedures may be indicated in this situation, often divided into soft tissue or bony procedures. Shoulder Instability – Soft Tissue: Surgical reconstruction targeting the glenohumeral joint’s soft tissues for shoulder instability, typically involves labral repairs, the most common being the Bankart repair. A Bankart lesion typically occurs from an anterior-inferior dislocation of the humerus, tearing the labrum from it’s attachment to the glenoid, thereby detaching the inferior gleno-humeral ligament (IGHL). Surgical management of this revolves around labral repair to reattach the IGHL under appropriate tension. This may be accomplished either arthroscopically or through an open approach.1 Most traumatic glenohumeral dislocations may not only cause a Bankart lesion, but may create impression fractures in the postero-superior humeral head termed Hill-Sachs lesions.2 An adverse effect from this procedure includes suturing the capsule too tightly, causing a shortening of the capsule, and thus decreasing the external rotation allowed at the glenohumeral joint. Other complications are extremely rare, but may include axillary nerve damage, subscapularis rupture (seen only in open repairs performed with subscapularis detachment and repair), and recurrent instability. If there is bony deficiency in the glenoid, which represents 20% or more of the antero-inferior glenoid, it is biomechanically impossible to restore the same stability and is therefore more prone to recurrent instability and failure. -

Coracoid Fractures and Dislocations
Coracoid Fractures and Dislocations Brett Gartrell Wildbase, Institute of Veterinary, Animal and Biomedical Sciences Massey University, Palmerston North, New Zealand ABSTRACT The treatment of coracoid dislocations and fractures in the New Zealand native wood pigeon (kereru; Hemiphaga novaeseelandiae), undertaken at the Wildbase Hospital at Massey University from 2002‐ 2012. After briefly reviewing the functional anatomy of the coracoid bones, the results of research on the nature of collisions that cause such injuries are reviewed. A comparative assessment of the relative force of collisions dependent on the body size of the bird is presented. The complications of coracoid fractures and dislocations including pulmonary haemorrhage, aspergillosis, cardiac contusions and rhythm disturbances is discussed. A new technique for the repair of coracoid dislocations from the keel and intramedullary pinning for repair of coracoid fractures are outlined. The merits of surgical versus conservative treatment of these injuries are discussed. ANATOMY The coracoid bones are essential to flight in birds and a reduced version of them is present in avian ancestors including Archaeopteryx, the earliest known flying bird from the Late Jurassic period (Figure 1) (Baier et al., 2007). Figure 1. Diagrammatic reconstruction of Archaeopteryx skeleton showing the modified coracoid bone (arrow) attached to the limited keel. Image modified from (Rowe, 2011). The function of the avian shoulder in flight is a complex interplay of forces from muscles, tendons and ligaments acting on the structural skeletal framework of coracoids, scapula, clavicles, humerus and keel. The coracoid bones are strut‐like support bones running from the shoulder to the keel, whose function is to resist the compressive forces of the pectoral muscle contraction (Figure 2).