On the Evolution of Bilaterality Grigory Genikhovich* and Ulrich Technau*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spicule Formation in Calcareous Sponges: Coordinated Expression
www.nature.com/scientificreports OPEN Spicule formation in calcareous sponges: Coordinated expression of biomineralization genes and Received: 17 November 2016 Accepted: 02 March 2017 spicule-type specific genes Published: 13 April 2017 Oliver Voigt1, Maja Adamska2, Marcin Adamski2, André Kittelmann1, Lukardis Wencker1 & Gert Wörheide1,3,4 The ability to form mineral structures under biological control is widespread among animals. In several species, specific proteins have been shown to be involved in biomineralization, but it is uncertain how they influence the shape of the growing biomineral and the resulting skeleton. Calcareous sponges are the only sponges that form calcitic spicules, which, based on the number of rays (actines) are distinguished in diactines, triactines and tetractines. Each actine is formed by only two cells, called sclerocytes. Little is known about biomineralization proteins in calcareous sponges, other than that specific carbonic anhydrases (CAs) have been identified, and that uncharacterized Asx-rich proteins have been isolated from calcitic spicules. By RNA-Seq and RNA in situ hybridization (ISH), we identified five additional biomineralization genes inSycon ciliatum: two bicarbonate transporters (BCTs) and three Asx-rich extracellular matrix proteins (ARPs). We show that these biomineralization genes are expressed in a coordinated pattern during spicule formation. Furthermore, two of the ARPs are spicule- type specific for triactines and tetractines (ARP1 orSciTriactinin ) or diactines (ARP2 or SciDiactinin). Our results suggest that spicule formation is controlled by defined temporal and spatial expression of spicule-type specific sets of biomineralization genes. By the process of biomineralization many animal groups produce mineral structures like skeletons, shells and teeth. Biominerals differ in shape considerably from their inorganic mineral counterparts1. -

Bryozoan Studies 2019
BRYOZOAN STUDIES 2019 Edited by Patrick Wyse Jackson & Kamil Zágoršek Czech Geological Survey 1 BRYOZOAN STUDIES 2019 2 Dedication This volume is dedicated with deep gratitude to Paul Taylor. Throughout his career Paul has worked at the Natural History Museum, London which he joined soon after completing post-doctoral studies in Swansea which in turn followed his completion of a PhD in Durham. Paul’s research interests are polymatic within the sphere of bryozoology – he has studied fossil bryozoans from all of the geological periods, and modern bryozoans from all oceanic basins. His interests include taxonomy, biodiversity, skeletal structure, ecology, evolution, history to name a few subject areas; in fact there are probably none in bryozoology that have not been the subject of his many publications. His office in the Natural History Museum quickly became a magnet for visiting bryozoological colleagues whom he always welcomed: he has always been highly encouraging of the research efforts of others, quick to collaborate, and generous with advice and information. A long-standing member of the International Bryozoology Association, Paul presided over the conference held in Boone in 2007. 3 BRYOZOAN STUDIES 2019 Contents Kamil Zágoršek and Patrick N. Wyse Jackson Foreword ...................................................................................................................................................... 6 Caroline J. Buttler and Paul D. Taylor Review of symbioses between bryozoans and primary and secondary occupants of gastropod -

Identical Genomic Organization of Two Hemichordate Hox Clusters
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Current Biology 22, 2053–2058, November 6, 2012 ª2012 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2012.08.052 Report Identical Genomic Organization of Two Hemichordate Hox Clusters Robert Freeman,1,12 Tetsuro Ikuta,2,12 Michael Wu,3 [10] have similar organization to the hemichordate cluster, Ryo Koyanagi,2 Takeshi Kawashima,2 Kunifumi Tagawa,4 but with different posterior genes. These results provide Tom Humphreys,5 Guang-Chen Fang,6 Asao Fujiyama,7 genomic evidence for a well-ordered complex in the deutero- Hidetoshi Saiga,8 Christopher Lowe,9 Kim Worley,10 stome ancestor for the hox1–hox9/10 region, with the Jerry Jenkins,11 Jeremy Schmutz,11 Marc Kirschner,1 number and kind of posterior genes still to be elucidated. Daniel Rokhsar,3 Nori Satoh,2,* and John Gerhart3,* 1 Department of Systems Biology, Harvard Medical School, Results and Discussion Boston, MA 02114, USA 2 Marine Genomics Unit, Okinawa Institute of Science and Here we characterize the order, transcriptional orientation, and Technology Graduate University, Onna, clustering of the Hox genes of the genomes of two widely Okinawa 904-0495, Japan studied model hemichordates, Saccoglossus kowalevskii 3 Department of Molecular and Cell Biology, University of and Ptychodera flava [4, 11, 12], that represent two major California, Berkeley, Berkeley, CA 94720-3200, USA evolutionary branches of enteropneust hemichordates that 4 Marine Biological Laboratory, Graduate School of Science, diverged an estimated 400 million years ago (MYa) [13]: Hiroshima University, Onomichi, Hiroshima 722-0073, Japan Saccoglossus, of the direct developing harimaniids, and 5 Department of Cell and Molecular Biology, University of Ptychodera, of the indirect developing ptychoderids [14]. -
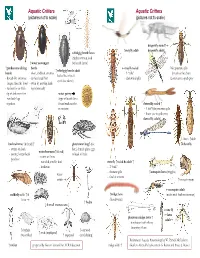
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Comparative Neuroanatomy of Mollusks and Nemerteans in the Context of Deep Metazoan Phylogeny
Comparative Neuroanatomy of Mollusks and Nemerteans in the Context of Deep Metazoan Phylogeny Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades einer Doktorin der Naturwissenschaften genehmigte Dissertation vorgelegt von Diplom-Biologin Simone Faller aus Frankfurt am Main Berichter: Privatdozent Dr. Rudolf Loesel Universitätsprofessor Dr. Peter Bräunig Tag der mündlichen Prüfung: 09. März 2012 Diese Dissertation ist auf den Internetseiten der Hochschulbibliothek online verfügbar. Contents 1 General Introduction 1 Deep Metazoan Phylogeny 1 Neurophylogeny 2 Mollusca 5 Nemertea 6 Aim of the thesis 7 2 Neuroanatomy of Minor Mollusca 9 Introduction 9 Material and Methods 10 Results 12 Caudofoveata 12 Scutopus ventrolineatus 12 Falcidens crossotus 16 Solenogastres 16 Dorymenia sarsii 16 Polyplacophora 20 Lepidochitona cinerea 20 Acanthochitona crinita 20 Scaphopoda 22 Antalis entalis 22 Entalina quinquangularis 24 Discussion 25 Structure of the brain and nerve cords 25 Caudofoveata 25 Solenogastres 26 Polyplacophora 27 Scaphopoda 27 i CONTENTS Evolutionary considerations 28 Relationship among non-conchiferan molluscan taxa 28 Position of the Scaphopoda within Conchifera 29 Position of Mollusca within Protostomia 30 3 Neuroanatomy of Nemertea 33 Introduction 33 Material and Methods 34 Results 35 Brain 35 Cerebral organ 38 Nerve cords and peripheral nervous system 38 Discussion 38 Peripheral nervous system 40 Central nervous system 40 In search for the urbilaterian brain 42 4 General Discussion 45 Evolution of higher brain centers 46 Neuroanatomical glossary and data matrix – Essential steps toward a cladistic analysis of neuroanatomical data 49 5 Summary 53 6 Zusammenfassung 57 7 References 61 Danksagung 75 Lebenslauf 79 ii iii 1 General Introduction Deep Metazoan Phylogeny The concept of phylogeny follows directly from the theory of evolution as published by Charles Darwin in The origin of species (1859). -

Development of the Annelid Axochord: Insights Into Notochord Evolution Antonella Lauri Et Al
RESEARCH | REPORTS ORIGIN OF NOTOCHORD by double WMISH (Fig. 2, F to L). Although none of the genes were exclusively expressed in the annelid mesodermal midline, their combined Development of the annelid coexpression was unique to these cells (implying that mesodermal midline in annelids and chor- damesoderm in vertebrates are more similar to axochord: Insights into each other than to any other tissue). It is unlikely that the molecular similarity between annelid notochord evolution and vertebrate mesodermal midline is due to in- dependent co-option of a conserved gene cas- Antonella Lauri,1*† Thibaut Brunet,1* Mette Handberg-Thorsager,1,2‡ sette, because this would require either that this Antje H.L. Fischer,1§ Oleg Simakov,1 Patrick R. H. Steinmetz,1‖ Raju Tomer,1,2¶ cassette was active elsewhere in the body (which Philipp J. Keller,2 Detlev Arendt1,3# is not the case) or that multiple identical inde- pendent events of co-option occurred (which is The origin of chordates has been debated for more than a century, with one key issue being unparsimonious). As in vertebrates, the meso- the emergence of the notochord. In vertebrates, the notochord develops by convergence dermal midline resembles the neuroectodermal and extension of the chordamesoderm, a population of midline cells of unique molecular midline, which expresses foxD, foxA, netrin, slit, identity. We identify a population of mesodermal cells in a developing invertebrate, the marine and noggin (figs. S6 and S7) but not brachyury or annelid Platynereis dumerilii, that converges and extends toward the midline and expresses a twist. However, unlike in chicken (10), the an- notochord-specific combination of genes. -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©1999 Academic Press. This manuscript is an author version with the final publication available and may be cited as: Young, C. M. (1999). Marine invertebrate larvae. In E. Knobil & J. D. Neill (eds.), Encyclopedia of Reproduction, 3. (pp. 89-97). London, England, and San Diego, CA: Academic Press. --------1111------- Marine Invertebrate Larvae Craig M. Young Harbor Branch Oceanographic Institution 1. What Is a Larva? metamorphOSiS Morphological and physiological changes II. The Production of Larvae that occur during the transition from the larval phase to iII. Larval forms and Diversity the juvenile phase: often coincides with settlement in ben IV. Larval Feeding and Nutrition thic species. V. Larval Orientation, Locomotion, Dispersal, and mixed development A developmental mode that includes a Mortality brooded or encapsulated embryonic stage as well as a free VI. Larval Settlement and Metamorphosis swimming larval stage. VlI. Ecological and Evolutionary Significance of Larvae planktotrophic larva A feeding larva that obtains at least part VlIl. Economic and Medical Importance of Larvae of its nutritional needs from either particulate or dissolved exogenous sources. Planktotrophic larvae generally hatch from small, transparent eggs. GLOSSARY settlement The permanent transition of a larva from the plankton to the benthos. In sessile organisms, settlement atrochal larva A uniformly ciliated larva (cilia not arranged is marked by adhesion to the substratum. It is often closely in distinct bands). associated with metamorphosis and may involve habitat se competent larva A larva that is physiologically and morpho lection. -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

University of Copenhagen, Zoological Museum, Review Universitetsparken 15, DK-2100 Copenhagen, Denmark CN, 0000-0001-6898-7655 Cite This Article: Nielsen C
Early animal evolution a morphologist's view Nielsen, Claus Published in: Royal Society Open Science DOI: 10.1098/rsos.190638 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Nielsen, C. (2019). Early animal evolution: a morphologist's view. Royal Society Open Science, 6(7), 1-10. [190638]. https://doi.org/10.1098/rsos.190638 Download date: 30. sep.. 2021 Early animal evolution: a morphologist’s view royalsocietypublishing.org/journal/rsos Claus Nielsen The Natural History Museum of Denmark, University of Copenhagen, Zoological Museum, Review Universitetsparken 15, DK-2100 Copenhagen, Denmark CN, 0000-0001-6898-7655 Cite this article: Nielsen C. 2019 Early animal evolution: a morphologist’s view. R. Soc. open sci. Two hypotheses for the early radiation of the metazoans are vividly discussed in recent phylogenomic studies, the ‘Porifera- 6: 190638. first’ hypothesis, which places the poriferans as the sister group http://dx.doi.org/10.1098/rsos.190638 of all other metazoans, and the ‘Ctenophora-first’ hypothesis, which places the ctenophores as the sister group to all other metazoans. It has been suggested that an analysis of morphological characters (including specific molecules) could Received: 5 April 2019 throw additional light on the controversy, and this is the aim of Accepted: 4 July 2019 this paper. Both hypotheses imply independent evolution of nervous systems in Planulozoa and Ctenophora. The Porifera- first hypothesis implies no homoplasies or losses of major characters. The Ctenophora-first hypothesis shows no important synapomorphies of Porifera, Planulozoa and Placozoa. It implies Subject Category: either independent evolution, in Planulozoa and Ctenophora, of Biology (whole organism) a new digestive system with a gut with extracellular digestion, which enables feeding on larger organisms, or the subsequent Subject Areas: loss of this new gut in the Poriferans (and the re-evolution of the evolution collar complex). -

Defining Phyla: Evolutionary Pathways to Metazoan Body Plans
EVOLUTION & DEVELOPMENT 3:6, 432-442 (2001) Defining phyla: evolutionary pathways to metazoan body plans Allen G. Collins^ and James W. Valentine* Museum of Paleontology and Department of Integrative Biology, University of California, Berkeley, CA 94720, USA 'Author for correspondence (email: [email protected]) 'Present address: Section of Ecology, Befiavior, and Evolution, Division of Biology, University of California, San Diego, La Jolla, CA 92093-0116, USA SUMMARY Phyla are defined by two sets of criteria, one pothesis of Nielsen; the clonal hypothesis of Dewel; the set- morphological and the other historical. Molecular evidence aside cell hypothesis of Davidson et al.; and a benthic hy- permits the grouping of animals into clades and suggests that pothesis suggested by the fossil record. It is concluded that a some groups widely recognized as phyla are paraphyletic, benthic radiation of animals could have supplied the ances- while some may be polyphyletic; the phyletic status of crown tral lineages of all but a few phyla, is consistent with molecu- phyla is tabulated. Four recent evolutionary scenarios for the lar evidence, accords well with fossil evidence, and accounts origins of metazoan phyla and of supraphyletic clades are as- for some of the difficulties in phylogenetic analyses of phyla sessed in the light of a molecular phylogeny: the trochaea hy- based on morphological criteria. INTRODUCTION Molecules have provided an important operational ad- vance to addressing questions about the origins of animal Concepts of animal phyla have changed importantly from phyla. Molecular developmental and comparative genomic their origins in the six Linnaean classis and four Cuvieran evidence offer insights into the genetic bases of body plan embranchements. -

A Phylum-Wide Survey Reveals Multiple Independent Gains of Head Regeneration Ability in Nemertea
bioRxiv preprint doi: https://doi.org/10.1101/439497; this version posted October 11, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. A phylum-wide survey reveals multiple independent gains of head regeneration ability in Nemertea Eduardo E. Zattara1,2,5, Fernando A. Fernández-Álvarez3, Terra C. Hiebert4, Alexandra E. Bely2 and Jon L. Norenburg1 1 Department of Invertebrate Zoology, National Museum of Natural History, Smithsonian Institution, Washington, DC, USA 2 Department of Biology, University of Maryland, College Park, MD, USA 3 Institut de Ciències del Mar, Consejo Superior de Investigaciones Científicas, Barcelona, Spain 4 Institute of Ecology and Evolution, University of Oregon, Eugene, OR, USA 5 INIBIOMA, Consejo Nacional de Investigaciones Científicas y Tecnológicas, Bariloche, RN, Argentina Corresponding author: E.E. Zattara, [email protected] Abstract Animals vary widely in their ability to regenerate, suggesting that regenerative abilities have a rich evolutionary history. However, our understanding of this history remains limited because regeneration ability has only been evaluated in a tiny fraction of species. Available comparative regeneration studies have identified losses of regenerative ability, yet clear documentation of gains is lacking. We surveyed regenerative ability in 34 species spanning the phylum Nemertea, assessing the ability to regenerate heads and tails either through our own experiments or from literature reports. Our sampling included representatives of the 10 most diverse families and all three orders comprising this phylum. -

Xenacoelomorpha's Significance for Understanding Bilaterian Evolution
Available online at www.sciencedirect.com ScienceDirect Xenacoelomorpha’s significance for understanding bilaterian evolution Andreas Hejnol and Kevin Pang The Xenacoelomorpha, with its phylogenetic position as sister biology models are the fruitfly Drosophila melanogaster and group of the Nephrozoa (Protostomia + Deuterostomia), plays the nematode Caenorhabditis elegans, in which basic prin- a key-role in understanding the evolution of bilaterian cell types ciples of developmental processes have been studied in and organ systems. Current studies of the morphological and great detail. It might be because the field of evolutionary developmental diversity of this group allow us to trace the developmental biology — EvoDevo — has its origin in evolution of different organ systems within the group and to developmental biology and not evolutionary biology that reconstruct characters of the most recent common ancestor of species under investigation are often called ‘model spe- Xenacoelomorpha. The disparity of the clade shows that there cies’. Criteria for selected representative species are cannot be a single xenacoelomorph ‘model’ species and primarily the ease of access to collected material and strategic sampling is essential for understanding the evolution their ability to be cultivated in the lab [1]. In some cases, of major traits. With this strategy, fundamental insights into the a supposedly larger number of ancestral characters or a evolution of molecular mechanisms and their role in shaping dominant role in ecosystems have played an additional animal organ systems can be expected in the near future. role in selecting model species. These arguments were Address used to attract sufficient funding for genome sequencing Sars International Centre for Marine Molecular Biology, University of and developmental studies that are cost-intensive inves- Bergen, Thormøhlensgate 55, 5008 Bergen, Norway tigations.