The Case of Brazilian Codling Urophycis Brasiliensis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reproductive Biology of Forkbeard, Phycis Phycis (Linnaeus, 1766) (Phycidae) in the Adriatic Sea (Croatia)
www.trjfas.org ISSN 1303-2712 Turkish Journal of Fisheries and Aquatic Sciences 14: 165-171 (2014) DOI: 10.4194/1303-2712-v14_1_18 The Reproductive Biology of Forkbeard, Phycis phycis (Linnaeus, 1766) (Phycidae) in the Adriatic Sea (Croatia) 1 2, 2 3 Katarina Glavić , Tatjana Dobroslavić *, Vlasta Bartulović , Sanja Matić-Skoko , Branko Glamuzina2 1 Private Secondary School Dubrovnik, Sustjepanska 4, 20000 Dubrovnik, Croatia. 2 University of Dubrovnik, Department of Aquaculture, Ćire Carića 4, 20000 Dubrovnik, Croatia. 3 Institute of Oceanography and Fisheries, Šetalište Ivana Meštrovića 63, P.O. Box 500, 21000 Split, Croatia. * Corresponding Author: Tel.: +385.20 445868; Fax: ; Received 20 November 2013 E-mail: [email protected] Accepted 24 January 2014 Abstract The forkbeard, Phycis phycis (Linnaeus, 1766), is a species widely distributed throughout the Mediterranean and Adriatic Sea and although it is of great importance for fishing industry, little is known about its reproductive characteristics. This study provides first data on reproductive characteristics of this species in the Mediterranean, important for management and stock assessment. For this purpose a total of 550 individuals ranging from 19.9 to 45.8 cm in total length were collected monthly in a period of one year using trammel nets. Sex ratio of males to females was 0.62:1. The estimated length where 50% of analysed individuals were sexually mature was 30.98 cm for females and 32.98 cm males. The peak value of gonadosomatic index was recorded in November and continued throughout the December, indicating the highest spawning activity. This period is characterized by presence of oocytes with migrated nucleus and yolk coalesces in ovary and spermatozoa in testes. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

MELANONIDAE Melanonus Zugmayeri Norman, 1930
click for previous page Gadiformes: Melanonidae 1001 MELANONIDAE Pelagic cods by T. Iwamoto, California Academy of Sciences, USA and D. M. Cohen, Bodega Bay, California, USA Melanonus zugmayeri Norman, 1930 En - Tropical pelagic cod. Diagnostic characters: Body slender, tapering to a narrow caudal peduncle. Head covered with free neuromasts aligned longitudinally into short ridges; pores of sensory lateralis system on head large, prominent; mouth large; teeth in 2 or 3 series in jaws, inner series laterally in lower jaw large, canine-like, widely spaced; teeth on vomer and palatines; no chin barbel. One long-based dorsal fin, high anteriorly, slightly notched at about sixth to tenth ray; anal fin long-based, rays finer than opposites of dorsal fin; caudal fin poorly developed, narrow, rounded to somewhat pointed; pectoral fin midlateral, below origin of dorsal fin; pelvic fin with 7 rays, origin anterior to pectoral base. Colour: overall blackish. Similar families occurring in the area Bathygadidae: no caudal fin; no teeth on roof of mouth; 2 separate dorsal fins, second ray of first dorsal fin a flexible spine, slightly to extremely prolonged. Gadidae: 2 or 3 separate dorsal fins; 1 or 2 anal fins; chin barbel present. 2nd ray is a 2 or 3 separate dorsal fins flexible 2 separate dorsal fins spine Bathygadidae chin Gadidae barbel Macrouridae: no caudal fin; no teeth on roof of mouth; scales usually covered with spinules. Moridae: 2 or 3 dorsal fins, 1or 2 anal fins, pelvic fins narrow with filamentous tips in some species; chin barbel developed in many; no enlarged, canine-like teeth in lower jaw, few or no teeth on vomer; swimbladder with an- terior projections that connect to rear of skull. -

Biodiversity of Arctic Marine Fishes: Taxonomy and Zoogeography
Mar Biodiv DOI 10.1007/s12526-010-0070-z ARCTIC OCEAN DIVERSITY SYNTHESIS Biodiversity of arctic marine fishes: taxonomy and zoogeography Catherine W. Mecklenburg & Peter Rask Møller & Dirk Steinke Received: 3 June 2010 /Revised: 23 September 2010 /Accepted: 1 November 2010 # Senckenberg, Gesellschaft für Naturforschung and Springer 2010 Abstract Taxonomic and distributional information on each Six families in Cottoidei with 72 species and five in fish species found in arctic marine waters is reviewed, and a Zoarcoidei with 55 species account for more than half list of families and species with commentary on distributional (52.5%) the species. This study produced CO1 sequences for records is presented. The list incorporates results from 106 of the 242 species. Sequence variability in the barcode examination of museum collections of arctic marine fishes region permits discrimination of all species. The average dating back to the 1830s. It also incorporates results from sequence variation within species was 0.3% (range 0–3.5%), DNA barcoding, used to complement morphological charac- while the average genetic distance between congeners was ters in evaluating problematic taxa and to assist in identifica- 4.7% (range 3.7–13.3%). The CO1 sequences support tion of specimens collected in recent expeditions. Barcoding taxonomic separation of some species, such as Osmerus results are depicted in a neighbor-joining tree of 880 CO1 dentex and O. mordax and Liparis bathyarcticus and L. (cytochrome c oxidase 1 gene) sequences distributed among gibbus; and synonymy of others, like Myoxocephalus 165 species from the arctic region and adjacent waters, and verrucosus in M. scorpius and Gymnelus knipowitschi in discussed in the family reviews. -
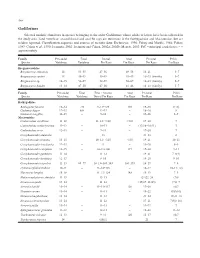
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

And Phycis Phycis (Linne, 1758), from the Western Algerian Coasts
Ukrainian Journal of Ecology, , 86-92, doi: 10.15421/2020_172 ORIGINAL ARTICLE Parasite diversity from two deep-sea fishes Phycis blennoides (Brunnich, 1768) and Phycis phycis (Linne, 1758), from the western Algerian coasts M.M. Hassani1, A. Kerfouf2, A. Baaloudj3*, F. Denis4 1Department of biology, Sciences Faculty, Saida University, 20000, Algeria 2,4Laboratoire d’écodéveloppement des espaces, Université Sidi Bel Abbes, 22000, Algeria 3Laboratoire Biologie, Eau et Environnement (LBEE). Faculté SNV-STU, Université 8 Mai 1945 Guelma BP.401 24000 Guelma, Algeria 4Département Milieux et Peuplements Aquatiques, UMR 7208 ‘BOREA’, Station de Biologie Marine du MNHN, Place de la Croix, 29182, Concarneau, France *Corresponding author E-mail: [email protected] Received: 04.08.2020. Accepted: 04.09.2020 This study investigated the diversity of metazoan parasites of 132 greater forkbeards and 91 forkbeards, from the western Algerian coasts, a total of 19 taxa: 15 taxa from Phycis blennoides; 11 taxa from Phycis phycis, allocated to 15 genera in 12 families were recovered, including 7species common to the two hosts. 90% are endoparasites, mostly located in the intestine and the stomach, encapsulate forms were in the liver, whereas crustaceans were found in the gills of the hosts. The Anisakidae Railliet & Henry, (1912) is the most diverse group with two genera: Hysterothylacium Ward & Magath, (1917) and Anisakis Dujardin, (1845) with 5 species. The nematode Hysterothylacium fabri (P= 55%) and the digenean Stephanostomum pristis are the most abundant and the most prevalent parasites from P. phycis (P=94%), while Hysterothylacium aduncum (P=49%) and Lepidapedon guevarai (P=87%) are from P. blennoides. 14 parasites species are present as adult stage, indicated that these fish act as their definitive hosts, while cosmopolite species are present in the larval stage. -

FIRST RECORD of Polymixia Nobilis LOWE, 1838 (ACTINOPTERYGII: POLYMIXIIDAE) in CEARÁ STATE, BRAZIL
FIRST RECORD OF Polymixia nobilis LOWE, 1838 (ACTINOPTERYGII: POLYMIXIIDAE) IN CEARÁ STATE, BRAZIL Primeiro registro de Polymixia nobilis Lowe, 1838 Arquivos de Ciências do Mar (Actinopterygii: Polymixiidae) no Estado do Ceará, Brasil Carolina Cerqueira de Paiva1, Rodrigo de Salles2, Maria Elisabeth de Araújo3 ABSTRACT The species Polymixia nobilis Lowe, 1838 (Actinopterygii: Polymixiidae) is recorded for the fi rst time in the state of Ceará, Brazil, based on one specimen collected on the Aracati Seamount at a 205 meter depth. Moreover, new meristic and morphologic data are presented. Key words: Polymixia nobilis, fi rst record, taxonomy, geographic distribution, Ceará State. RESUMO A espécie Polyxia nobilis Lowe, 1838 (Actinopterygii: Polimixiidae) é registrada pela promeira vez no Ceará devido à coleta de um exemplar no banco oceânico do Aracati a 205 m de profundidade. São apresentadas também novas informações sobre as variações merísticas e morfológicas da espécie. Palavras-chaves: Polymixia nobilis, primeiro registro, taxonomia, distribuição geográfi ca, Estado do Ceará. 1 Bióloga, Instituto de Cièncias do Mar, Laboratório de Ecologia Animal (LECA). Av. da Abolição 3207, Fortaleza, CE 60165-081. 2 Bolsista da Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico – CAPES/FUNCAP. 3 Professora Associada II, Departamento de Oceanografia – UFPE, Av. Arquitetura, s/n, Campus Universitário, Recife, PE 50740-550. Arq. Ciên. Mar, Fortaleza, 2009, 42(2): 39 - 42 39 INTRODUCTION The aim of the present study was to register the broadening of the geographic distribution of P. Polymixia is the only genus of family nobilis in northeastern Brazil. Polymixiidae and comprises ten species, among which only two are recorded in the Atlantic Ocean: MATERIALS AND METHODS Polymixia nobilis Lowe, 1838 and Polymixia lowei Günther 1859 (Eschmeyer, 2009), commonly known The material was obtained through surveys as beardfi sh (Woods & Sonoda, 1973). -

And Phycis (Artedi) Larvae and Pelagic Juveniles in the Middle Atlantic Bight
W&M ScholarWorks Dissertations, Theses, and Masters Projects Theses, Dissertations, & Master Projects 1987 Identification and Distribution of Urophycis (Gill) and Phycis (Artedi) Larvae and Pelagic Juveniles in the Middle Atlantic Bight Bruce Henry Comyns College of William and Mary - Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/etd Part of the Fresh Water Studies Commons, Marine Biology Commons, and the Oceanography Commons Recommended Citation Comyns, Bruce Henry, "Identification and Distribution of Urophycis (Gill) and Phycis (Artedi) Larvae and Pelagic Juveniles in the Middle Atlantic Bight" (1987). Dissertations, Theses, and Masters Projects. Paper 1539617573. https://dx.doi.org/doi:10.25773/v5-qgj5-7r45 This Thesis is brought to you for free and open access by the Theses, Dissertations, & Master Projects at W&M ScholarWorks. It has been accepted for inclusion in Dissertations, Theses, and Masters Projects by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. IDENTIFICATION AND DISTRIBUTION OF UROPHYCIS (Gill) AND PHYCIS (Artedi) LARVAE AND PELAGIC JUVENILES IN THE MIDDLE ATLANTIC BIGHT A Thesis Presented to The Faculty of the School of Marine Science The College of William and Mary in Virginia In Partial Fulfillment of the Requirements for the Degree of Master of Arts By Bruce H. Comyns 1987 LIBRARY \ of the This thesis is submitted in partial fulfillment of the requirements for the degree of Master of Arts f / ' Bruce H. Cornyns Approved, Ma.y 1987 George C^Grant, Ph.D. Committee Chairman/Advisor c. Johr Olney, M.A. ick, PhrD. Herbert^. Austin, Ph.D. -

The Intermuscular Bones and Ligaments of Teleostean Fishes *
* The Intermuscular Bones and Ligaments of Teleostean Fishes COLIN PATTERSON and G. DAVID JOHNSON m I I SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 559 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

EN615 Cruise Report
ADEON Recovery/Deployment Cruise Report #EN615 - RV Endeavor 06 – 25 June 2018 San Juan, Puerto Rico to Narragansett, RI Chief Scientist Joseph Warren, Jennifer Miksis-Olds, Carmen Lawrence, Brandyn Lucca, Hannah Blair, Sebastian Velez, Cassandra Fries, Peter Larios, Madison Alstede, Stephen Ell, 1 Jennifer Conyers, Andrew Heaney, Lindsay Olson, and Katharine Coykendall Cruise Summary The objectives for this cruise were to recover bottom landers at seven sites (Figure 1) along the shelfbreak (depths ranging from 200 – 900 m roughly), redeploy a bottom lander at each site after downloading its data, collect CTD profiles to characterize hydrographic conditions at the sites, conduct net sampling to collect biological specimens at each site, and conduct fine-scale (roughly 8 km by 8 km) multi-frequency acoustic surveys at each site (Figure 2). All cruise objectives were completed safely. In addition, we collected animal specimens from net tows for collaborators associated with the DEEP SEARCH project as well as collecting water samples for eDNA analysis for DEEP SEARCH and other collaborators. Water samples were also collected by a UNH undergraduate in support of her capstone paper. We were fortunate to have good weather for much of the cruise which allowed us to complete additional net tows, CTD, and fine-scale acoustic surveys at some sites (Table 1). We appreciate the excellent work of the ship’s Captain and crew (in all aspects on the boat) in helping us to accomplish our cruise objectives. Table 1. Summary of sampling that occurred at each site location during the EN615 research cruise. We were able to accomplish additional sampling at the VAC and HAT sites. -

Are Parasites of Phycis Blennoides (Gadiformes: Phycidae) Good Indicators of Metal Pollution in the Western Mediterranean Deep- Sea? C
Are parasites of Phycis blennoides (Gadiformes: Phycidae) good indicators of metal pollution in the Western Mediterranean deep- sea? C. M. Moyà-Alcover1, M. Nachev2, S. Zimmermann2, F. E. Montero1,3, M. Carrassón1 B. Sures2 and A. Pérez-del-Olmo1,2,3* 1Departament de Biologia Animal, de Biologia Vegetal i d'Ecologia, Universitat Autònoma de Barcelona, 08193 Cerdanyola del Vallès, Barcelona, Spain 2 Institute of Biology, Zoology/Hydrobiology, University Duisburg-Essen, Universitätsstr. 5, D- 45141 Essen, Germany 3 Unitat de Zoologia Marina, Institut Cavanilles de Biodiversitat i Biologia Evolutiva, Parc Científic, Universitat de València, Aptdo 22085, E-46071-Valencia, Spain * Presenting author contact: [email protected] In the last years the general concern about the significance of trace elements in the environment has rapidly increased. The alteration of the global cycles of trace elements as a consequence of a growing anthropogenic activity has enhanced natural concentrations of these elements in the marine environment, especially in coastal areas. But these contaminants can affect not only the littoral sediments but also deeper areas due to its transportation together with particulate matter. While coastal regions are relatively well characterised from a pollution perspective, the deep-sea areas are relatively unknown. A variety of organisms have been investigated to evaluate their potential as biological indicators of different forms of pollution in the aquatic environment and some fish parasites has been demonstrated to be useful because of their ability to bioconcentrate these pollutants even at much higher levels than free-living organisms. However, few studies have been carried out in the marine environment and studies from deep-sea are virtually lacking. -

HANDBOOK of FISH BIOLOGY and FISHERIES Volume 1 Also Available from Blackwell Publishing: Handbook of Fish Biology and Fisheries Edited by Paul J.B
HANDBOOK OF FISH BIOLOGY AND FISHERIES Volume 1 Also available from Blackwell Publishing: Handbook of Fish Biology and Fisheries Edited by Paul J.B. Hart and John D. Reynolds Volume 2 Fisheries Handbook of Fish Biology and Fisheries VOLUME 1 FISH BIOLOGY EDITED BY Paul J.B. Hart Department of Biology University of Leicester AND John D. Reynolds School of Biological Sciences University of East Anglia © 2002 by Blackwell Science Ltd a Blackwell Publishing company Chapter 8 © British Crown copyright, 1999 BLACKWELL PUBLISHING 350 Main Street, Malden, MA 02148‐5020, USA 108 Cowley Road, Oxford OX4 1JF, UK 550 Swanston Street, Carlton, Victoria 3053, Australia The right of Paul J.B. Hart and John D. Reynolds to be identified as the Authors of the Editorial Material in this Work has been asserted in accordance with the UK Copyright, Designs, and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs, and Patents Act 1988, without the prior permission of the publisher. First published 2002 Reprinted 2004 Library of Congress Cataloging‐in‐Publication Data has been applied for. Volume 1 ISBN 0‐632‐05412‐3 (hbk) Volume 2 ISBN 0‐632‐06482‐X (hbk) 2‐volume set ISBN 0‐632‐06483‐8 A catalogue record for this title is available from the British Library. Set in 9/11.5 pt Trump Mediaeval by SNP Best‐set Typesetter Ltd, Hong Kong Printed and bound in the United Kingdom by TJ International Ltd, Padstow, Cornwall.