And Phycis Phycis (Linne, 1758), from the Western Algerian Coasts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Twenty Thousand Parasites Under The
ADVERTIMENT. Lʼaccés als continguts dʼaquesta tesi queda condicionat a lʼacceptació de les condicions dʼús establertes per la següent llicència Creative Commons: http://cat.creativecommons.org/?page_id=184 ADVERTENCIA. El acceso a los contenidos de esta tesis queda condicionado a la aceptación de las condiciones de uso establecidas por la siguiente licencia Creative Commons: http://es.creativecommons.org/blog/licencias/ WARNING. The access to the contents of this doctoral thesis it is limited to the acceptance of the use conditions set by the following Creative Commons license: https://creativecommons.org/licenses/?lang=en Departament de Biologia Animal, Biologia Vegetal i Ecologia Tesis Doctoral Twenty thousand parasites under the sea: a multidisciplinary approach to parasite communities of deep-dwelling fishes from the slopes of the Balearic Sea (NW Mediterranean) Tesis doctoral presentada por Sara Maria Dallarés Villar para optar al título de Doctora en Acuicultura bajo la dirección de la Dra. Maite Carrassón López de Letona, del Dr. Francesc Padrós Bover y de la Dra. Montserrat Solé Rovira. La presente tesis se ha inscrito en el programa de doctorado en Acuicultura, con mención de calidad, de la Universitat Autònoma de Barcelona. Los directores Maite Carrassón Francesc Padrós Montserrat Solé López de Letona Bover Rovira Universitat Autònoma de Universitat Autònoma de Institut de Ciències Barcelona Barcelona del Mar (CSIC) La tutora La doctoranda Maite Carrassón Sara Maria López de Letona Dallarés Villar Universitat Autònoma de Barcelona Bellaterra, diciembre de 2016 ACKNOWLEDGEMENTS Cuando miro atrás, al comienzo de esta tesis, me doy cuenta de cuán enriquecedora e importante ha sido para mí esta etapa, a todos los niveles. -

The Reproductive Biology of Forkbeard, Phycis Phycis (Linnaeus, 1766) (Phycidae) in the Adriatic Sea (Croatia)
www.trjfas.org ISSN 1303-2712 Turkish Journal of Fisheries and Aquatic Sciences 14: 165-171 (2014) DOI: 10.4194/1303-2712-v14_1_18 The Reproductive Biology of Forkbeard, Phycis phycis (Linnaeus, 1766) (Phycidae) in the Adriatic Sea (Croatia) 1 2, 2 3 Katarina Glavić , Tatjana Dobroslavić *, Vlasta Bartulović , Sanja Matić-Skoko , Branko Glamuzina2 1 Private Secondary School Dubrovnik, Sustjepanska 4, 20000 Dubrovnik, Croatia. 2 University of Dubrovnik, Department of Aquaculture, Ćire Carića 4, 20000 Dubrovnik, Croatia. 3 Institute of Oceanography and Fisheries, Šetalište Ivana Meštrovića 63, P.O. Box 500, 21000 Split, Croatia. * Corresponding Author: Tel.: +385.20 445868; Fax: ; Received 20 November 2013 E-mail: [email protected] Accepted 24 January 2014 Abstract The forkbeard, Phycis phycis (Linnaeus, 1766), is a species widely distributed throughout the Mediterranean and Adriatic Sea and although it is of great importance for fishing industry, little is known about its reproductive characteristics. This study provides first data on reproductive characteristics of this species in the Mediterranean, important for management and stock assessment. For this purpose a total of 550 individuals ranging from 19.9 to 45.8 cm in total length were collected monthly in a period of one year using trammel nets. Sex ratio of males to females was 0.62:1. The estimated length where 50% of analysed individuals were sexually mature was 30.98 cm for females and 32.98 cm males. The peak value of gonadosomatic index was recorded in November and continued throughout the December, indicating the highest spawning activity. This period is characterized by presence of oocytes with migrated nucleus and yolk coalesces in ovary and spermatozoa in testes. -

Phycis Blennoides (Brünnich, 1768)
Phycis blennoides (Brünnich, 1768) AphiaID: 126501 ABRÓTEA-DO-ALTO Animalia (Reino) > Chordata (Filo) > Vertebrata (Subfilo) > Gnathostomata (Infrafilo) > Pisces (Superclasse) > Pisces (Superclasse-2) > Actinopterygii (Classe) > Gadiformes (Ordem) > Phycidae (Familia) © Diogo Rocha Claire Goodwin - iNaturalist.ca Descrição Corpo fusiforme; raios das barbatanas pélvicas estendem-se para além da origem da barbatana anal; 1º raio da barbatana dorsal é alongado; cor acastanhada ou acinzentada dorsalmente e mais clara ventralmente. Distribuição geográfica Atlântico nordeste, Mediterrâneo e Adriático. Habitat e ecologia Ocorre em zonas de fundos arenosos ou lamacentos, a profundidades que variam entre 150-300m. 1 Facilmente confundível com: Phycis phycis Abrótea-da-costa Estatuto de Conservação Sinónimos Batrachoides gmelini Risso, 1810 Blennius gadoides Lacepède, 1800 Gadus albidus Gmelin, 1789 Gadus bifurcus Walbaum, 1792 Gadus blennoides Brünnich, 1768 Phycis furcatus Fleming, 1828 Phycis tinca Bloch & Schneider, 1801 Urophycis blennioides (Brünnich, 1768) Informação Adicional Pesquise mais sobre Phycis blennoides> ~ FishBase ~Marine Species Identification Portal 2 Referências Manual Prático de Identificação de Peixes Ósseos da Costa Continental Portuguesa – IPMA (2018) additional source Wheeler, A. (1992). A list of the common and scientific names of fishes of the British Isles. J. Fish Biol. 41(Suppl. A): 1-37 [details] additional source Brünnich, M.T. (1768). Ichthyologia Massiliensis, sistens piscium descriptiones eorumque apud incolas nomina. Accedunt Spolia Maris Adriatici. Hafniae et Lipsiae. i-xvi + 1-110. [In 2 parts; first as i-xvi + 1-84; 2nd as Spolia e Mari Adriatica reportata: 85-110.]. [details] additional source Froese, R. & D. Pauly (Editors). (2017). FishBase. World Wide Web electronic publication. , available online at http://www.fishbase.org [details] basis of record van der Land, J.; Costello, M.J.; Zavodnik, D.; Santos, R.S.; Porteiro, F.M.; Bailly, N.; Eschmeyer, W.N.; Froese, R. -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

MELANONIDAE Melanonus Zugmayeri Norman, 1930
click for previous page Gadiformes: Melanonidae 1001 MELANONIDAE Pelagic cods by T. Iwamoto, California Academy of Sciences, USA and D. M. Cohen, Bodega Bay, California, USA Melanonus zugmayeri Norman, 1930 En - Tropical pelagic cod. Diagnostic characters: Body slender, tapering to a narrow caudal peduncle. Head covered with free neuromasts aligned longitudinally into short ridges; pores of sensory lateralis system on head large, prominent; mouth large; teeth in 2 or 3 series in jaws, inner series laterally in lower jaw large, canine-like, widely spaced; teeth on vomer and palatines; no chin barbel. One long-based dorsal fin, high anteriorly, slightly notched at about sixth to tenth ray; anal fin long-based, rays finer than opposites of dorsal fin; caudal fin poorly developed, narrow, rounded to somewhat pointed; pectoral fin midlateral, below origin of dorsal fin; pelvic fin with 7 rays, origin anterior to pectoral base. Colour: overall blackish. Similar families occurring in the area Bathygadidae: no caudal fin; no teeth on roof of mouth; 2 separate dorsal fins, second ray of first dorsal fin a flexible spine, slightly to extremely prolonged. Gadidae: 2 or 3 separate dorsal fins; 1 or 2 anal fins; chin barbel present. 2nd ray is a 2 or 3 separate dorsal fins flexible 2 separate dorsal fins spine Bathygadidae chin Gadidae barbel Macrouridae: no caudal fin; no teeth on roof of mouth; scales usually covered with spinules. Moridae: 2 or 3 dorsal fins, 1or 2 anal fins, pelvic fins narrow with filamentous tips in some species; chin barbel developed in many; no enlarged, canine-like teeth in lower jaw, few or no teeth on vomer; swimbladder with an- terior projections that connect to rear of skull. -

Biodiversity of Arctic Marine Fishes: Taxonomy and Zoogeography
Mar Biodiv DOI 10.1007/s12526-010-0070-z ARCTIC OCEAN DIVERSITY SYNTHESIS Biodiversity of arctic marine fishes: taxonomy and zoogeography Catherine W. Mecklenburg & Peter Rask Møller & Dirk Steinke Received: 3 June 2010 /Revised: 23 September 2010 /Accepted: 1 November 2010 # Senckenberg, Gesellschaft für Naturforschung and Springer 2010 Abstract Taxonomic and distributional information on each Six families in Cottoidei with 72 species and five in fish species found in arctic marine waters is reviewed, and a Zoarcoidei with 55 species account for more than half list of families and species with commentary on distributional (52.5%) the species. This study produced CO1 sequences for records is presented. The list incorporates results from 106 of the 242 species. Sequence variability in the barcode examination of museum collections of arctic marine fishes region permits discrimination of all species. The average dating back to the 1830s. It also incorporates results from sequence variation within species was 0.3% (range 0–3.5%), DNA barcoding, used to complement morphological charac- while the average genetic distance between congeners was ters in evaluating problematic taxa and to assist in identifica- 4.7% (range 3.7–13.3%). The CO1 sequences support tion of specimens collected in recent expeditions. Barcoding taxonomic separation of some species, such as Osmerus results are depicted in a neighbor-joining tree of 880 CO1 dentex and O. mordax and Liparis bathyarcticus and L. (cytochrome c oxidase 1 gene) sequences distributed among gibbus; and synonymy of others, like Myoxocephalus 165 species from the arctic region and adjacent waters, and verrucosus in M. scorpius and Gymnelus knipowitschi in discussed in the family reviews. -

Relatório E Contas, Ano 2020
RELATÓRIO E CONTAS PROJETAR A INVESTIGAÇÃO CIENTÍFICA PARA RESPONDER AO DESAFIO DO SÉCULO XXI - VIVER BEM DENTRO DOS LIMITES DO PLANETA 2020 IPMA, IP - RELATÓRIO DE ATIVIDADES 2020 ÍNDICE 1. NOTA INTRODUTÓRIA ............................................................................................................................................................. 4 2. ATIVIDADES REALIZADAS ........................................................................................................................................................ 7 2.1 GESTÃO ............................................................................................................................................................................ 7 2.1.1 GESTÃO FINANCEIRA ................................................................................................................................................ 7 2.1.2 GESTÃO DE RECURSOS HUMANOS ........................................................................................................................... 7 2.1.3 GESTÃO DE CONTRATOS .......................................................................................................................................... 8 2.1.4 GESTÃO DE INFRAESTRUTURAS GENÉRICAS ............................................................................................................ 9 2.1.5 GESTÃO DE INFRAESTRUTURAS DE IT E SUPERCOMPUTAÇÃO .............................................................................. 10 2.1.6 GESTÃO DE PRODUTOS, SERVIÇOS E -
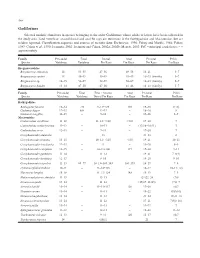
Gadiformes Selected Meristic Characters in Species Belonging to the Order Gadiformes Whose Adults Or Larvae Have Been Collected in the Study Area
548 Gadiformes Selected meristic characters in species belonging to the order Gadiformes whose adults or larvae have been collected in the study area. Total vertebrae, second dorsal and anal fin rays are numerous in the Bathygadidae and Macrouridae, but are seldom reported. Classification sequence and sources of meristic data: Eschmeyer, 1990; Fahay and Markle, 1984; Fahay, 1989; Cohen et al., 1990; Iwamoto, 2002; Iwamoto and Cohen, 2002a; 2002b; Merrett, 2003. PrC = principal caudal rays; ~ = approximately Family Precaudal Total Dorsal Anal Pectoral Pelvic Species Vertebrae Vertebrae Fin Rays Fin Rays Fin Rays Fin Rays Bregmacerotidae Bregmaceros atlanticus 14 53–55 47–56 49–58 16–21 5–7 Bregmaceros cantori 14 45–49 45–49 45–49 16–23 (family) 5–7 Bregmaceros sp. 14–15 52–59 52–59 58–69 16–23 (family) 5–7 Bregmaceros houdei 13–14 47–50 47–50 41–46 16–23 (family) 5–7 Family Precaudal Total First + Second Anal Pectoral Pelvic Species Vertebrae Vertebrae Dorsal Fin Rays Fin Rays Fin Rays Fin Rays Bathygadidae Bathygadus favosus 12–14 ~70 9–11+125 110 15–18 9(10) Gadomus dispar 12–13 80+ 12–13 – 18–20 8 Gadomus longifilis 11–13 – 9–11 – 14–16 8–9 Macrouridae Caelorinchus caribbeus 11–12 – 11–12+>110 >110 17–20 7 Caelorinchus coelorhynchus 11–12 – 10–11 – (17)18–20(21) 7 Caelorinchus occa 12–13 – 9–11 – 17–20 7 Coryphaenoides alateralis – 13 – 21–23 8 Coryphaenoides armatus 13–15 – 10–12+~125 ~135 19–21 10–11 Coryphaenoides brevibarbis 12–13 – 9 – 19–20 8–9 Coryphaenoides carapinus 12–15 – 10–11+100 117 17–20 9–11 Coryphaenoides guentheri -

Ahlem ROMDHANI 1, 2*, Mohamed-Hédi KTARI 1, Jean-Louis DUFOUR 2, Kélig MAHE 2, and Patrice FRANCOUR 3
ACTA ICHTHYOLOGICA ET PISCATORIA (2016) 46 (1): 25–32 DOI: 10.3750/AIP2016.46.1.08 GROWTH AND AGE ESTIMATION OF THE GREATER FORKBEARD, PHYCIS BLENNOIDES (ACTINOPTERYGII: GADIFORMES: PHYCIDAE), FROM THE GULF OF TUNIS (CENTRAL MEDITERRANEAN) Ahlem ROMDHANI 1, 2*, Mohamed-Hédi KTARI 1, Jean-Louis DUFOUR 2, Kélig MAHE 2, and Patrice FRANCOUR 3 1Laboratoire de biologie et biodiversité des populations, Faculté des sciences, Campus universitaire El Manar II, Tunis, Tunisia 2Institut Français de Recherche pour l’exploitation de la mer (IFREMER), Boulogne-sur-Mer, France 3 Université Nice Sophia Antipolis, CNRS, ECOMERS, Nice, France Romdhani A., Ktari M.-H., Dufour J.-L., Mahe K., Francour P. 2016. Growth and age estimation of the greater forkbeard, Phycis blennoides (Actinopterygii: Gadiformes: Phycidae), from the Gulf of Tunis (central Mediterranean). Acta Ichthyol. Piscat. 46 (1): 25–32. Background. The greater forkbeard, Phycis blennoides (Brünnich, 1768), is a gadiform species, which has economical value and its population is dwelling in a 60–800 m depth range throughout the Atlantic and the Mediterranean. Along African north coast, there is a lack of information about the biology (age, growth, sex-ratio) of this species. This study provides the fi rst data on population parameters of P. blennoides in Tunisia. Materials and methods. Specimens of Phycis blennoides were collected from the landings of the artisanal fi sheries between September 2007 and June 2010. The length–weight relations were determined according to the allometric equation: W = aTLb. Growth parameters were estimated using the von Bertalanffy growth equation. Results. Length (TL)–weight (W) relations were allometrically positive for the females (W = 22.10–4TL3.409), males (W = 13.10–4TL3.674), and both sexes (W = 19.10–4TL3.460), without signifi cant differences between males and females. -

Reproductive Biology and Growth of Greater Forkbeard Phycis Blennoides (Brünnich, 1768) in Western Algerian Coasts (Osteichthyes, Gadidae)
J. Bio. & Env. Sci. 2014 Journal of Biodiversity and Environmental Sciences (JBES) ISSN: 2220-6663 (Print) 2222-3045 (Online) Vol. 4, No. 6, p. 389-398, 2014 http://www.innspub.net RESEARCH PAPER OPEN ACCESS Reproductive biology and growth of greater forkbeard Phycis blennoides (Brünnich, 1768) in Western Algerian Coasts (Osteichthyes, Gadidae) Sofiane Med el Amine Benghali1&2*, Salim Mouffok2, Ali Kherraz1&2, Zitouni Boutiba2 1Department of Marine Science and Aquaculture, FSNV-UMAB-Mostaganem, Algeria 2Environmental Surveillance Laboratory, Department of Biology, Faculty of natural sciences and life, University Oran, BP 1524 El Menaouar, Algeria Article published on June 18, 2014 Key words: gonadosomatic index, hepatosomatic index, first maturity, spawning period. Abstract The Great forkbeard, Phycis blennoides (Brünnich, 1768), is a species widely distributed throughout the Mediterranean Sea and although it is of great importance for fishing industry, little is known about its reproductive characteristics. This study provides first data on reproductive characteristics of this species in the south western Mediterranean, important for management and stock assessment. For this purpose a total of 461 individuals ranging from 17.5 to 43.5 cm in total length were collected monthly in a period of one year using trammel nets. Sex ratio of males to females was 1:0.91. The estimated length where 50% of analyzed individuals were sexually mature was 24.73 cm for females. The peak value of gonad somatic index was recorded in September and continued throughout the October, indicating the highest spawning activity when the Kn values are low. *Corresponding Author: Sofiane Med el Amine Benghali [email protected] 389 | Benghali et al J. -

The Intermuscular Bones and Ligaments of Teleostean Fishes *
* The Intermuscular Bones and Ligaments of Teleostean Fishes COLIN PATTERSON and G. DAVID JOHNSON m I I SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 559 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review.