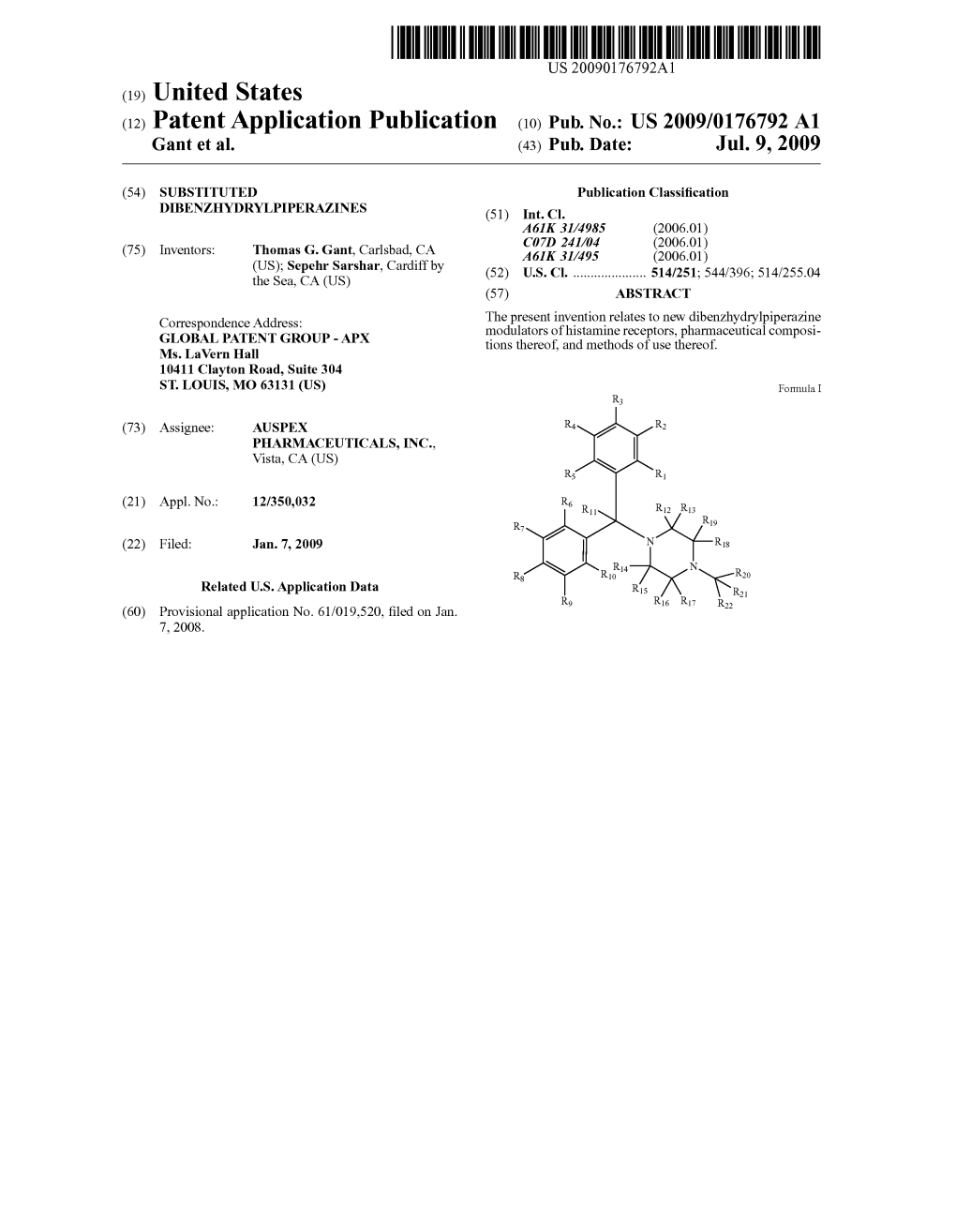
(12) Patent Application Publication (10) Pub. No.: US 2009/0176792 A1 Gant Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Update on Diagnosis and Treatment of Idiopathic Pulmonary Fibrosis
J Bras Pneumol. 2015;41(5):454-466 http://dx.doi.org/10.1590/S1806-37132015000000152 REVIEW ARTICLE Update on diagnosis and treatment of idiopathic pulmonary fibrosis José Baddini-Martinez1, Bruno Guedes Baldi2, Cláudia Henrique da Costa3, Sérgio Jezler4, Mariana Silva Lima5, Rogério Rufino3,6 1. Divisão de Pneumologia, Departamento de Clínica Médica, Faculdade de Medicina de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brasil. 2. Divisão de Pneumologia, Instituto do Coração, Hospital das Clínicas, ABSTRACT Faculdade de Medicina, Universidade de São Paulo, São Paulo, Brasil. Idiopathic pulmonary fibrosis is a type of chronic fibrosing interstitial pneumonia, of 3. Disciplina de Pneumologia e Tisiologia, unknown etiology, which is associated with a progressive decrease in pulmonary Faculdade de Ciências Médicas, function and with high mortality rates. Interest in and knowledge of this disorder have Universidade do Estado do Rio de grown substantially in recent years. In this review article, we broadly discuss distinct Janeiro, Rio de Janeiro, Brasil. aspects related to the diagnosis and treatment of idiopathic pulmonary fibrosis. We 4. Ambulatório de Pneumologia, Hospital list the current diagnostic criteria and describe the therapeutic approaches currently Ana Nery, Salvador, Brasil. available, symptomatic treatments, the action of new drugs that are effective in slowing 5. Ambulatório de Doenças Pulmonares Intersticiais, Hospital do Servidor the decline in pulmonary function, and indications for lung transplantation. Público Estadual de São Paulo, São Keywords: Idiopathic pulmonary fibrosis/diagnosis; Idiopathic pulmonary fibrosis/therapy; Paulo, Brasil. Idiopathic pulmonary fibrosis/rehabilitation. 6. Programa de Pós-Graduação em Ciências Médicas, Universidade do Estado do Rio de Janeiro, Rio de Janeiro, Brasil. -

Guidelines for the Forensic Analysis of Drugs Facilitating Sexual Assault and Other Criminal Acts
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Forensic analysis of drugs facilitating sexual assault and other criminal acts United Nations publication Printed in Austria ST/NAR/45 *1186331*V.11-86331—December 2011 —300 Photo credits: UNODC Photo Library, iStock.com/Abel Mitja Varela Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Guidelines for the forensic analysis of drugs facilitating sexual assault and other criminal acts UNITED NATIONS New York, 2011 ST/NAR/45 © United Nations, December 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. List of abbreviations . v Acknowledgements .......................................... vii 1. Introduction............................................. 1 1.1. Background ........................................ 1 1.2. Purpose and scope of the manual ...................... 2 2. Investigative and analytical challenges ....................... 5 3 Evidence collection ...................................... 9 3.1. Evidence collection kits .............................. 9 3.2. Sample transfer and storage........................... 10 3.3. Biological samples and sampling ...................... 11 3.4. Other samples ...................................... 12 4. Analytical considerations .................................. 13 4.1. Substances encountered in DFSA and other DFC cases .... 13 4.2. Procedures and analytical strategy...................... 14 4.3. Analytical methodology .............................. 15 4.4. -

Product List March 2019 - Page 1 of 53
Wessex has been sourcing and supplying active substances to medicine manufacturers since its incorporation in 1994. We supply from known, trusted partners working to full cGMP and with full regulatory support. Please contact us for details of the following products. Product CAS No. ( R)-2-Methyl-CBS-oxazaborolidine 112022-83-0 (-) (1R) Menthyl Chloroformate 14602-86-9 (+)-Sotalol Hydrochloride 959-24-0 (2R)-2-[(4-Ethyl-2, 3-dioxopiperazinyl) carbonylamino]-2-phenylacetic 63422-71-9 acid (2R)-2-[(4-Ethyl-2-3-dioxopiperazinyl) carbonylamino]-2-(4- 62893-24-7 hydroxyphenyl) acetic acid (r)-(+)-α-Lipoic Acid 1200-22-2 (S)-1-(2-Chloroacetyl) pyrrolidine-2-carbonitrile 207557-35-5 1,1'-Carbonyl diimidazole 530-62-1 1,3-Cyclohexanedione 504-02-9 1-[2-amino-1-(4-methoxyphenyl) ethyl] cyclohexanol acetate 839705-03-2 1-[2-Amino-1-(4-methoxyphenyl) ethyl] cyclohexanol Hydrochloride 130198-05-9 1-[Cyano-(4-methoxyphenyl) methyl] cyclohexanol 93413-76-4 1-Chloroethyl-4-nitrophenyl carbonate 101623-69-2 2-(2-Aminothiazol-4-yl) acetic acid Hydrochloride 66659-20-9 2-(4-Nitrophenyl)ethanamine Hydrochloride 29968-78-3 2,4 Dichlorobenzyl Alcohol (2,4 DCBA) 1777-82-8 2,6-Dichlorophenol 87-65-0 2.6 Diamino Pyridine 136-40-3 2-Aminoheptane Sulfate 6411-75-2 2-Ethylhexanoyl Chloride 760-67-8 2-Ethylhexyl Chloroformate 24468-13-1 2-Isopropyl-4-(N-methylaminomethyl) thiazole Hydrochloride 908591-25-3 4,4,4-Trifluoro-1-(4-methylphenyl)-1,3-butane dione 720-94-5 4,5,6,7-Tetrahydrothieno[3,2,c] pyridine Hydrochloride 28783-41-7 4-Chloro-N-methyl-piperidine 5570-77-4 -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

(12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 De Juan Et Al
US 200601 10428A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2006/0110428A1 de Juan et al. (43) Pub. Date: May 25, 2006 (54) METHODS AND DEVICES FOR THE Publication Classification TREATMENT OF OCULAR CONDITIONS (51) Int. Cl. (76) Inventors: Eugene de Juan, LaCanada, CA (US); A6F 2/00 (2006.01) Signe E. Varner, Los Angeles, CA (52) U.S. Cl. .............................................................. 424/427 (US); Laurie R. Lawin, New Brighton, MN (US) (57) ABSTRACT Correspondence Address: Featured is a method for instilling one or more bioactive SCOTT PRIBNOW agents into ocular tissue within an eye of a patient for the Kagan Binder, PLLC treatment of an ocular condition, the method comprising Suite 200 concurrently using at least two of the following bioactive 221 Main Street North agent delivery methods (A)-(C): Stillwater, MN 55082 (US) (A) implanting a Sustained release delivery device com (21) Appl. No.: 11/175,850 prising one or more bioactive agents in a posterior region of the eye so that it delivers the one or more (22) Filed: Jul. 5, 2005 bioactive agents into the vitreous humor of the eye; (B) instilling (e.g., injecting or implanting) one or more Related U.S. Application Data bioactive agents Subretinally; and (60) Provisional application No. 60/585,236, filed on Jul. (C) instilling (e.g., injecting or delivering by ocular ion 2, 2004. Provisional application No. 60/669,701, filed tophoresis) one or more bioactive agents into the Vit on Apr. 8, 2005. reous humor of the eye. Patent Application Publication May 25, 2006 Sheet 1 of 22 US 2006/0110428A1 R 2 2 C.6 Fig. -

ERJ-01090-2018.Supplement
Shaheen et al Online data supplement Prescribed analgesics in pregnancy and risk of childhood asthma Seif O Shaheen, Cecilia Lundholm, Bronwyn K Brew, Catarina Almqvist. 1 Shaheen et al Figure E1: Data available for analysis Footnote: Numbers refer to adjusted analyses (complete data on covariates) 2 Shaheen et al Table E1. Three classes of analgesics included in the analyses ATC codes Generic drug name Opioids N02AA59 Codeine, combinations excluding psycholeptics N02AA79 Codeine, combinations with psycholeptics N02AA08 Dihydrocodeine N02AA58 Dihydrocodeine, combinations N02AC04 Dextropropoxyphene N02AC54 Dextropropoxyphene, combinations excluding psycholeptics N02AX02 Tramadol Anti-migraine N02CA01 Dihydroergotamine N02CA02 Ergotamine N02CA04 Methysergide N02CA07 Lisuride N02CA51 Dihydroergotamine, combinations N02CA52 Ergotamine, combinations excluding psycholeptics N02CA72 Ergotamine, combinations with psycholeptics N02CC01 Sumatriptan N02CC02 Naratriptan N02CC03 Zolmitriptan N02CC04 Rizatriptan N02CC05 Almotriptan N02CC06 Eletriptan N02CC07 Frovatriptan N02CX01 Pizotifen N02CX02 Clonidine N02CX03 Iprazochrome N02CX05 Dimetotiazine N02CX06 Oxetorone N02CB01 Flumedroxone Paracetamol N02BE01 Paracetamol N02BE51 Paracetamol, combinations excluding psycholeptics N02BE71 Paracetamol, combinations with psycholeptics 3 Shaheen et al Table E2. Frequency of analgesic classes prescribed to the mother during pregnancy Opioids Anti- Paracetamol N % migraine No No No 459,690 93.2 No No Yes 9,091 1.8 Yes No No 15,405 3.1 No Yes No 2,343 0.5 Yes No -

Pharmacokinetics, Pharmacodynamics and Drug
pharmaceutics Review Pharmacokinetics, Pharmacodynamics and Drug–Drug Interactions of New Anti-Migraine Drugs—Lasmiditan, Gepants, and Calcitonin-Gene-Related Peptide (CGRP) Receptor Monoclonal Antibodies Danuta Szkutnik-Fiedler Department of Clinical Pharmacy and Biopharmacy, Pozna´nUniversity of Medical Sciences, Sw.´ Marii Magdaleny 14 St., 61-861 Pozna´n,Poland; [email protected] Received: 28 October 2020; Accepted: 30 November 2020; Published: 3 December 2020 Abstract: In the last few years, there have been significant advances in migraine management and prevention. Lasmiditan, ubrogepant, rimegepant and monoclonal antibodies (erenumab, fremanezumab, galcanezumab, and eptinezumab) are new drugs that were launched on the US pharmaceutical market; some of them also in Europe. This publication reviews the available worldwide references on the safety of these anti-migraine drugs with a focus on the possible drug–drug (DDI) or drug–food interactions. As is known, bioavailability of a drug and, hence, its pharmacological efficacy depend on its pharmacokinetics and pharmacodynamics, which may be altered by drug interactions. This paper discusses the interactions of gepants and lasmiditan with, i.a., serotonergic drugs, CYP3A4 inhibitors, and inducers or breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp) inhibitors. In the case of monoclonal antibodies, the issue of pharmacodynamic interactions related to the modulation of the immune system functions was addressed. It also focuses on the effect of monoclonal antibodies on expression of class Fc gamma receptors (FcγR). Keywords: migraine; lasmiditan; gepants; monoclonal antibodies; drug–drug interactions 1. Introduction Migraine is a chronic neurological disorder characterized by a repetitive, usually unilateral, pulsating headache with attacks typically lasting from 4 to 72 h. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Curriculum 2015/2016
218 Curriculum 2015/2016 5th year / spring semester The deadline of submitting the thesis is March 10, 2016 Pharmacy students perform a 4-month clerkship (3 successive months in public pharmacies, 1 month can be accomplished in pharmacy, pharmaceutical factory, galenical laboratory, university department or hospital pharmacy). Fees: From the academic year 2005/2006 fifth year pharmacy students have to pay the whole tuition fee for the second semester of the fifth year. INTERIM PRACTICE Pharmacy students are required to complete a compulsory practice in a pharmacy which must be accredited by the country concerned. At the completion of the practice an evaluation form should be filled in, signed, stamped and sent directly from the pharmacy or submitted by the student in a sealed envelope. (The form can be downloaded from our website). A “Letter of Acceptance ” completed by the pharmacy has to be presented at the Foreign Students’ Secretariat until May, 2016 . 2nd year pharmacy students must perform a practice of 4 weeks in a pharmacy. 3rd year pharmacy students must perform a practice of 4 weeks in a pharmacy. 4th year pharmacy students must perform a practice of 2 months in a pharmacy (pharmacy, pharmaceutical factory, galenical laboratory, university department or hospital/clinical pharmacy). Note: The precondition of starting the 2-month compulsory practice is completing all the courses of the first four years and acquiring 16 credits of elective subjects. 5th year pharmacy students must perform a 4-month clerkship in the second semester of the academic year. (3 successive months in public pharmacies and 1 month in a hospital/clinical pharmacy.) Curriculum 2015/2016 219 SYLLABUSES FOR 1ST YEAR PHARMACY STUDENTS PHYSICS-BIOPHYSICS 1st semester LECTURE Flow of fluids. -

Genl:VE 1970 © World Health Organization 1970
Nathan B. Eddy, Hans Friebel, Klaus-Jiirgen Hahn & Hans Halbach WORLD HEALTH ORGANIZATION ORGANISATION .MONDIALE DE LA SANT~ GENl:VE 1970 © World Health Organization 1970 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. Nevertheless governmental agencies or learned and professional societies may reproduce data or excerpts or illustrations from them without requesting an authorization from the World Health Organization. For rights of reproduction or translation of WHO publications in toto, application should be made to the Division of Editorial and Reference Services, World Health Organization, Geneva, Switzerland. The World Health Organization welcomes such applications. Authors alone are responsible for views expressed in signed articles. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Director-General of the World Health Organization concerning the legal status of any country or territory or of its authorities, or concerning the delimitation of its frontiers. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. © Organisation mondiale de la Sante 1970 Les publications de l'Organisation mondiale de la Sante beneficient de la protection prevue par les dispositions du Protocole n° 2 de la Convention universelle pour la Protection du Droit d'Auteur. Les institutions gouvernementales et les societes savantes ou professionnelles peuvent, toutefois, reproduire des donnees, des extraits ou des illustrations provenant de ces publications, sans en demander l'autorisation a l'Organisation mondiale de la Sante. Pour toute reproduction ou traduction integrate, une autorisation doit etre demandee a la Division des Services d'Edition et de Documentation, Organisation mondiale de la Sante, Geneve, Suisse. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO).