Torus Mandibularis: Etiology and Bioarcheological Utility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lumps and Swellings
Clinical Oral medicine for the general practitioner: lumps and swellings Crispian Scully 1 his series of five papers summarises some of the most important oral medicine problems likely to be Tencountered by practitioners. Some are common, others rare. The practitioner cannot be expected to diagnose all, but has been trained to recognise oral health and disease, and should be competent to recognise normal variants, and common orofacial disorders. In any case of doubt, the practitioner is advised to seek a second opinion from a colleague. The series is not intended to be comprehensive in coverage either of the conditions encountered, or all aspects of Figure 1: Torus mandibularis. diagnosis or treatment: further details are available in standard texts, in the further reading section, or from the internet. The present article discusses aspects of lumps through fear, perhaps after hearing of someone with and swellings. ‘mouth cancer’. Thus some individuals discover and worry about normal anatomical features such as tori, the parotid Lumps and swellings papilla, foliate papillae on the tongue, or the pterygoid Lumps and swellings in the mouth are common, but of hamulus. The tongue often detects even a very small diverse aetiologies (Table 1), and some represent swelling, or the patient may first notice it because it is sore malignant neoplasms. Therefore, this article will discuss (Figure 1). In contrast, many oral cancers are diagnosed far lumps and swellings in general terms, but later focus on too late, often after being present several months, usually the particular problems of oral cancer and of orofacial because the patient ignores the swelling. -

Glossary for Narrative Writing
Periodontal Assessment and Treatment Planning Gingival description Color: o pink o erythematous o cyanotic o racial pigmentation o metallic pigmentation o uniformity Contour: o recession o clefts o enlarged papillae o cratered papillae o blunted papillae o highly rolled o bulbous o knife-edged o scalloped o stippled Consistency: o firm o edematous o hyperplastic o fibrotic Band of gingiva: o amount o quality o location o treatability Bleeding tendency: o sulcus base, lining o gingival margins Suppuration Sinus tract formation Pocket depths Pseudopockets Frena Pain Other pathology Dental Description Defective restorations: o overhangs o open contacts o poor contours Fractured cusps 1 ww.links2success.biz [email protected] 914-303-6464 Caries Deposits: o Type . plaque . calculus . stain . matera alba o Location . supragingival . subgingival o Severity . mild . moderate . severe Wear facets Percussion sensitivity Tooth vitality Attrition, erosion, abrasion Occlusal plane level Occlusion findings Furcations Mobility Fremitus Radiographic findings Film dates Crown:root ratio Amount of bone loss o horizontal; vertical o localized; generalized Root length and shape Overhangs Bulbous crowns Fenestrations Dehiscences Tooth resorption Retained root tips Impacted teeth Root proximities Tilted teeth Radiolucencies/opacities Etiologic factors Local: o plaque o calculus o overhangs 2 ww.links2success.biz [email protected] 914-303-6464 o orthodontic apparatus o open margins o open contacts o improper -

Supernumerary Teeth
Volume 22, Number 1, 2009 ISSN 1096-9411 Dental Anthropology A Publication of the Dental Anthropology Association Dental Anthropology Volume 22, Number 1, 2009 Dental Anthropology is the Official Publication of the Dental Anthropology Association. Editor: Edward F. Harris Editorial Board Kurt W. Alt (2004-2009) Richard T. Koritzer (2004-2009) A. M. Haeussler (2004-2009) Helen Liversidge (2004-2009) Tseunehiko Hanihara (2004-2009) Yuji Mizoguchi (2006-2010) Kenneth A. R. Kennedy (2006-2010) Lindsay C. Richards (2006-2010) Jules A. Kieser (2004-2009) Phillip W. Walker (2006-2010) Officers of the Dental Anthropology Association Brian E. Hemphill (California State University, Bakersfield) President (2008-2010) G. Richard Scott (University of Nevada, Reno) President-Elect (2008-2010) Loren R. Lease (Youngstown State University, Ohio) Secretary-Treasurer (2007-2009) Simon W. Hillson (University College London) Past-President (2006-2008) Address for Manuscripts Dr. Edward F. Harris College of Dentistry, University of Tennessee 870 Union Avenue, Memphis, TN 38163 U.S.A. E-mail address: [email protected] Address for Book Reviews Dr. Greg C. Nelson Department of Anthropology, University of Oregon Condon Hall, Eugene, Oregon 97403 U.S.A. E-mail address: [email protected] Published at Craniofacial Biology Laboratory, Department of Orthodontics College of Dentistry, The Health Science Center University of Tennessee, Memphis, TN 38163 U.S.A. The University of Tennessee is an EEO/AA/Title IX/Section 504/ADA employer 1 Strong genetic influence on hypocone expression of permanent maxillary molars in South Australian twins Denice Higgins*, Toby E. Hughes, Helen James, Grant C. Townsend Craniofacial Biology Research Group, School of Dentistry, The University of Adelaide, South Australia 5005 ABSTRACT An understanding of the role of genetic be larger in males although this was not statistically influences on dental traits is important in the areas of significant. -

WHAT HAPPENED? CDR, a 24-Year-Old Chinese Male
CHILDHOOD DEVELOPMENTAL SCREENING 2020 https://doi.org/10.33591/sfp.46.5.up1 FINDING A MASS WITHIN THE ORAL CAVITY: WHAT ARE THE COMMON CAUSES AND 4-7 GAINING INSIGHT: WHAT ARE THE ISSUES? In Figure 2 below, a list of masses that could arise from each site Figure 3. Most common oral masses What are the common salivary gland pathologies Salivary gland tumours (Figure 7) commonly present as channel referrals to appropriate specialists who are better HOW SHOULD A GP MANAGE THEM? of the oral cavity is given and elaborated briey. Among the that a GP should be aware of? painless growing masses which are usually benign. ey can equipped in centres to accurately diagnose and treat these Mr Tan Tai Joum, Dr Marie Stella P Cruz CDR had a slow-growing mass in the oral cavity over one year more common oral masses are: torus palatinus, torus occur in both major and minor salivary glands but are most patients, which usually involves surgical excision. but sought treatment only when he experienced a sudden acute mandibularis, pyogenic granuloma, mucocele, broma, ere are three pairs of major salivary glands (parotid, commonly found occurring in the parotid glands. e most 3) Salivary gland pathology may be primary or secondary to submandibular and sublingual) as well as hundreds of minor ABSTRACT onset of severe pain and numbness. He was fortunate to have leukoplakia and squamous cell carcinoma – photographs of common type of salivary gland tumour is the pleomorphic systemic causes. ese dierent diseases may present with not sought treatment as it had not caused any pain. -
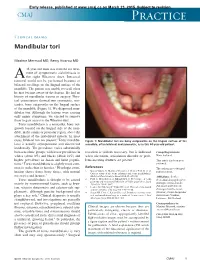
Mandibular Tori
Early release, published at www.cmaj.ca on March 23, 2015. Subject to revision. CMAJ Practice Clinical images Mandibular tori Maxime Mermod MD, Remy Hoarau MD 44-year-old man was referred for treat- ment of symptomatic sialolithiasis in A the right Wharton duct. Intraoral removal could not be performed because of bilateral swellings on the lingual surface of the mandible. The patient was unable to recall when he first became aware of the lesions. He had no history of mandibular trauma or surgery. Phys- ical examination showed two symmetric, non- tender, bony outgrowths on the lingual surface of the mandible (Figure 1). We diagnosed man- dibular tori. Although the lesions were causing only minor symptoms, we elected to remove them to gain access to the Wharton duct. Torus mandibularis is a nontender, bony out- growth located on the lingual side of the man- dible, in the canine or premolar region, above the attachment of the mylohyoid muscle. In most 1 cases, bilateral tori are present. Torus mandibu- Figure 1: Mandibular tori are bony outgrowths on the lingual surface of the laris is usually asymptomatic and discovered mandible, often bilateral and symmetric, as in this 44-year-old patient. incidentally. The prevalence varies substantially between ethnic groups, with lower prevalence in resection is seldom necessary, but is indicated Competing interests: whites (about 8%) and blacks (about 16%) and when ulceration, articulation disorder or prob- None declared. higher prevalence in Asian and Inuit popula- lems inserting dentures are present.3 This article has been peer tions.2 Torus mandibularis is slightly more com- reviewed. -

Abscesses Apicectomy
BChD, Dip Odont. (Mondchir.) MBChB, MChD (Chir. Max.-Fac.-Med.) Univ. of Pretoria Co Reg: 2012/043819/21 Practice.no: 062 000 012 3323 ABSCESSES WHAT IS A TOOTH ABSCESS? A dental/tooth abscess is a localised acute infection at the base of a tooth, which requires immediate attention from your dentist. They are usually associated with acute pain, swelling and sometimes an unpleasant smell or taste in the mouth. More severe infections cause facial swelling as the bacteria spread to the nearby tissues of the face. This is a very serious condition. Once the swelling begins, it can spread rapidly. The pain is often made worse by drinking hot or cold fluids or biting on hard foods and may spread from the tooth to the ear or jaw on the same side. WHAT CAUSES AN ABSCESS? Damage to the tooth, an untreated cavity, or a gum disease can cause an abscessed tooth. If the cavity isn’t treated, the inside of the tooth can become infected. The bacteria can spread from the tooth to the tissue around and beneath it, creating an abscess. Gum disease causes the gums to pull away from the teeth, leaving pockets. If food builds up in one of these pockets, bacteria can grow, and an abscess may form. An abscess can cause the bone around the tooth to dissolve. WHY CAN'T ANTIBIOTIC TREATMENT ALONE BE USED? Antibiotics will usually help the pain and swelling associated with acute dental infections. However, they are not very good at reaching into abscesses and killing all the bacteria that are present. -

Recognition and Management of Oral Health Problems in Older Adults by Physicians: a Pilot Study
J Am Board Fam Pract: first published as 10.3122/jabfm.11.6.474 on 1 November 1998. Downloaded from BRIEF REPORTS Recognition and Management of Oral Health Problems in Older Adults by Physicians: A Pilot Study Thomas V. Jones, MD, MPH, Mitchel J Siegel, DDS, andJohn R. Schneider, A1A Oral health problems are among the most com of the nation's current and future health care mon chronic health conditions experienced by needs, the steady increase in the older adult popu older adults. Healthy People 2000, an initiative to lation, and the generally high access elderly per improve the health of America, has selected oral sons have to medical care provided by family health as a priority area. l About 11 of 100,000 physicians and internists.s,7,8 Currently there is persons have oral cancer diagnosed every year.2 very little information about the ability of family The average age at which oral cancer is diagnosed physicians or internists, such as geriatricians, to is approximately 65 years, with the incidence in assess the oral health of older patients. We con creasing from middle adulthood through the sev ducted this preliminary study to determine how enth decade of life. l-3 Even though the mortality family physicians and geriatricians compare with rate associated with oral cancer (7700 deaths an each other and with general practice dentists in nually)4 ranks among the lowest compared with their ability to recognize, diagnose, and perform other cancers, many deaths from oral cancer initial management of a wide spectrum of oral might be prevented by improved case finding and health problems seen in older adults. -

Comunicaciones Pósteres
1113-5181/18/26.1/59-101 ODONTOLOGÍA PEDIÁTRICA ODONTOL PEDIÁTR (Madrid) COPYRIGHT © 2018 SEOP Y ARÁN EDICIONES, S. L. Vol. 26, N.º 1, pp. 59-101, 2018 Comunicaciones Pósteres REVISIÓN BIBLIOGRÁFICA resulta imperativo que los pediatras incrementen su nivel de conocimiento sobre la CPI y faciliten más información eficaz a los padres sobre cuidados orales y la necesidad de visitar al odontopediatra. Los padres poseen escasos conocimientos sobre la caries, especialmente acerca de su tratamiento. 0002. CONOCIMIENTO DE PEDIATRAS Y PADRES SOBRE LA CARIES DE LA PRIMERA INFANCIA Enrech Rivero, J.; Sande López, L.; Martínez 0012. ENFERMEDAD CELIACA Y ALTERACIONES Martín, N.; Martín Olivera, E.; Delgado Castro, N. DEL ESMALTE DENTAL. REVISIÓN SISTEMÁTICA Universidad Antonio de Nebrija. Madrid López Durán, M.; Riolobos González, M.; Introducción: La prevalencia universal de la caries es un Costa Ferrer, F.; Khalifi Abdelkader, C.; recordatorio constante de la necesidad de proporcionar una edu- de la Cuesta Aubert, A. cación eficaz para la prevención en la salud oral. La caries de la Universidad Alfonso X el Sabio. Villanueva de la Cañada, primera infancia (CPI) es una enfermedad infecciosa, crónica Madrid y transmisible, con una etiología multifactorial, considerada actualmente un grave problema de salud pública universal en Introducción: La enfermedad celíaca (EC) es una enfer- niños en edad escolar. Los datos epidemiológicos muestran medad sistémica inmunomediada, provocada por el gluten que la mejor manera de controlar la CPI se basa precisamente y prolaminas, en individuos genéticamente susceptibles; se en la prevención, que en el niño consistirá en actuar sobre los caracteriza por la presencia de una combinación variable de factores etiológicos, como mejorar los hábitos dietéticos e hi- manifestaciones clínicas dependientes del gluten, anticuerpos giénicos. -

Oral Pathology Final Exam Review Table Tuanh Le & Enoch Ng, DDS
Oral Pathology Final Exam Review Table TuAnh Le & Enoch Ng, DDS 2014 Bump under tongue: cementoblastoma (50% 1st molar) Ranula (remove lesion and feeding gland) dermoid cyst (neoplasm from 3 germ layers) (surgical removal) cystic teratoma, cyst of blandin nuhn (surgical removal down to muscle, recurrence likely) Multilocular radiolucency: mucoepidermoid carcinoma cherubism ameloblastoma Bump anterior of palate: KOT minor salivary gland tumor odontogenic myxoma nasopalatine duct cyst (surgical removal, rare recurrence) torus palatinus Mixed radiolucencies: 4 P’s (excise for biopsy; curette vigorously!) calcifying odontogenic (Gorlin) cyst o Pyogenic granuloma (vascular; granulation tissue) periapical cemento-osseous dysplasia (nothing) o Peripheral giant cell granuloma (purple-blue lesions) florid cemento-osseous dysplasia (nothing) o Peripheral ossifying fibroma (bone, cartilage/ ossifying material) focal cemento-osseous dysplasia (biopsy then do nothing) o Peripheral fibroma (fibrous ct) Kertocystic Odontogenic Tumor (KOT): unique histology of cyst lining! (see histo notes below); 3 important things: (1) high Multiple bumps on skin: recurrence rate (2) highly aggressive (3) related to Gorlin syndrome Nevoid basal cell carcinoma (Gorlin syndrome) Hyperparathyroidism: excess PTH found via lab test Neurofibromatosis (see notes below) (refer to derm MD, tell family members) mucoepidermoid carcinoma (mixture of mucus-producing and squamous epidermoid cells; most common minor salivary Nevus gland tumor) (get it out!) -

Oral Health and Disease
Downloaded from bmj.com on 19 August 2005 ABC of oral health: Oral health and disease Ruth Holt, Graham Roberts and Crispian Scully BMJ 2000;320;1652-1655 doi:10.1136/bmj.320.7250.1652 Updated information and services can be found at: http://bmj.com/cgi/content/full/320/7250/1652 These include: Rapid responses One rapid response has been posted to this article, which you can access for free at: http://bmj.com/cgi/content/full/320/7250/1652#responses You can respond to this article at: http://bmj.com/cgi/eletter-submit/320/7250/1652 Email alerting Receive free email alerts when new articles cite this article - sign up in the box at service the top right corner of the article Topic collections Articles on similar topics can be found in the following collections Dentistry and Oral Medicine (79 articles) Notes To order reprints of this article go to: http://www.bmjjournals.com/cgi/reprintform To subscribe to BMJ go to: http://bmj.bmjjournals.com/subscriptions/subscribe.shtml Clinical review Downloaded from bmj.com on 19 August 2005 ABC of oral health Oral health and disease Ruth Holt, Graham Roberts, Crispian Scully A healthy dentition and mouth is important to both quality of life and nutrition, and oral disease may affect systemic health, as Enamel covering crown Gingival crevice discussed in later articles in this series. (gingival sulcus) Dentine Development of the dentition Gingiva Pulp chamber Teeth form mainly from neuroectoderm and comprise a crown of insensitive enamel surrounding sensitive dentine and a root Periodontal ligament that has no enamel covering. -

QUICK ORAL HEALTH FACTS ABOUT the YOUNG Dr Ng Jing Jing, Dr Wong Mun Loke
ORAL health IN PRIMARY CARE UNIT NO. 2 QUICK ORAL HEALTH FACTS ABOUT THE YOUNG Dr Ng Jing Jing, Dr Wong Mun Loke ABSTRACT Table 1. Eruption sequence of Primary Dentition This article sheds light on the sequence of teeth eruption Primary Upper Teeth Primary Lower Teeth in the young and teething problems; highlights the importance and functions of the primary dentition and Central Incisors: 8-13 months Central Incisors: 6-10 months provides a quick overview of common developmental Lateral Incisors: 8-13 months Lateral Incisors: 10-16 months dental anomalies and other dental conditions in Canines: 16-23 months Canines: 16-23 months children. First Molars: 16-23 months First Molars: 13-19 months Second Molars: 25-33 months Second Molars: 23-31 months SFP2011; 37(1) Supplement : 10-13 Table 2. Eruption sequence of Adult Dentition Adult Upper Teeth Adult Lower Teeth INTRODUCTION Central Incisors: 7-8 years Central Incisors: 6-7 years The early years are always full of exciting moments as we observe Lateral Incisors: 8-9 years Lateral Incisors: 7-8 years our children grow and develop. One of the most noticeable Canines: 11-12 years Canines: 9-10 years aspects of their growth and development is the eruption of First Premolars: 10-11 years First Premolars: 10-11 years teeth. The first sign of a tooth in the mouth never fails to Second Premolars: 11-12 years Second Premolars: 11-12 years attract the attention of the parent and child. For the parent, it First Molars: 6-7 years First Molars: 6-7 years marks an important developmental milestone of the child but Second Molars: 12-13 years Second Molars: 11-13 years for the child, it can be a source of irritation brought on by the Third Molars: 18-25 years Third Molars: 18-25 years whole process of teething. -

Concurrence of Torus Palatinus, Torus Mandibularis and Buccal Exostosis Sarfaraz Khan1, Syed Asif Haider Shah2, Farman Ali3 and Dil Rasheed4
CASE REPORT Concurrence of Torus Palatinus, Torus Mandibularis and Buccal Exostosis Sarfaraz Khan1, Syed Asif Haider Shah2, Farman Ali3 and Dil Rasheed4 ABSTRACT Torus palatinus (TP), torus mandibularis (TM), and buccal exostosis are localised, benign, osseous projections, occurring in maxilla and mandible. Etiology is multifactorial and not well established. Tori and exostoses have been associated with parafunctional occlusal habits, temporomandibular joint (TMJ) disorders, migraine and consumption of fish. Concurrence of TP, TM, and exostosis in the same individual is very rare. Concurrence of TP and TM has not been reported from Pakistan. We report a case of a 22-year female patient manifesting concurrence of TP, bilateral TM, and maxillary buccal exostoses; with possible association of abnormal occlusal stresses and use of calcium and vitamin D supplements. Key Words: Torus palatinus. Torus mandibularis. Exostoses. INTRODUCTION upper teeth, for the last one year. She noticed a gradual Torus palatinus (TP) is a localised, benign, osseous increase in the severity of her symptoms. The patient projection in midline of the hard palate. Torus denied any associated pain or ulceration. She had mandibularis (TM) is a benign, bony protuberance, on remained under orthodontic treatment for 2 years for the lingual aspect of the mandible, usually bilaterally, at correction of her crooked teeth. After completion of the the canine-premolar area, above the mylohyoid line. treatment, she was advised to wear removable retainer appliance; but owing to her admittedly non-compliant Exostoses are multiple small bony nodules occurring attitude towards treatment, malalignment of her teeth along the buccal or palatal aspects of maxilla and buccal recurred within the next 2 years.