Transitional Tholeiitic Basalts in the Tertiary Bana Volcano–Plutonic Complex, Cameroon Line
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Source to Surface Model of Monogenetic Volcanism: a Critical Review
Downloaded from http://sp.lyellcollection.org/ by guest on September 28, 2021 Source to surface model of monogenetic volcanism: a critical review I. E. M. SMITH1 &K.NE´ METH2* 1School of Environment, University of Auckland, Auckland, New Zealand 2Volcanic Risk Solutions, Massey University, Palmerston North 4442, New Zealand *Correspondence: [email protected] Abstract: Small-scale volcanic systems are the most widespread type of volcanism on Earth and occur in all of the main tectonic settings. Most commonly, these systems erupt basaltic magmas within a wide compositional range from strongly silica undersaturated to saturated and oversatu- rated; less commonly, the spectrum includes more siliceous compositions. Small-scale volcanic systems are commonly monogenetic in the sense that they are represented at the Earth’s surface by fields of small volcanoes, each the product of a temporally restricted eruption of a composition- ally distinct batch of magma, and this is in contrast to polygenetic systems characterized by rela- tively large edifices built by multiple eruptions over longer periods of time involving magmas with diverse origins. Eruption styles of small-scale volcanoes range from pyroclastic to effusive, and are strongly controlled by the relative influence of the characteristics of the magmatic system and the surface environment. Gold Open Access: This article is published under the terms of the CC-BY 3.0 license. Small-scale basaltic magmatic systems characteris- hazards associated with eruptions, and this is tically occur at the Earth’s surface as fields of small particularly true where volcanic fields are in close monogenetic volcanoes. These volcanoes are the proximity to population centres. -

Geochemistry of Sideromelane and Felsic Glass Shards in Pleistocene Ash Layers at Sites 953, 954, and 9561
Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., and Duffield, W. (Eds.), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 157 25. GEOCHEMISTRY OF SIDEROMELANE AND FELSIC GLASS SHARDS IN PLEISTOCENE ASH LAYERS AT SITES 953, 954, AND 9561 Andrey A. Gurenko2 and Hans-Ulrich Schmincke2 ABSTRACT Sideromelane and felsic glass shards from unconsolidated Pleistocene volcaniclastic sediments drilled at Sites 953, 954, and 956 are thought to have derived from submarine and subaerial volcanic eruptions on Gran Canaria (Sites 953 and 954) and Tenerife (Sites 954 and 956). We analyzed these glasses by electron microprobe for major elements and sulfur, chlorine, and fluorine. Sideromelane glasses represent a spectrum from alkali basalt through basanite, hawaiite, mugearite, and tephrite to nephelinite. Felsic glasses have compositions similar to benmoreite, trachyte, and phonolite. Vesiculated mafic and felsic glass shards, which are characterized by low S and Cl concentrations (0.01−0.06 wt% S and 0.01–0.04 wt% Cl), are interpreted to have formed by pyroclastic activity on land or in shallow water and appeared to have been strongly degassed. Vesicle-free blocky glass shards having 0.05−0.13 wt% S are likely to have resulted from submarine eruptions at moderate water depths and represent undegassed or slightly degassed magmas. Cl concentrations range from 0.01 to 0.33 wt% and increase with increasing MgO, suggesting that Cl behaves as an incompatible element during magma crystallization. Concentrations of fluorine (0.04− 0.34 wt% F) are likely to represent undegassed values, and the variations in F/K ratios between 0.02 and 0.24 are believed to reflect those of parental magmas and of the mantle source. -

31 a Preliminary Study Of
31 A PRELIMINARY STUDY OF THE: TERTIARY VOLCANIC AND SEDIMENTARY ROCKS, GÜMELE, ESKİŞEHİR Eskişehir, Gümele Çevresindeki Tersiyer Volkanik ve Sedimanter Kayaçlarda Bir Ön Çalışma Taylan Lünel Middle East Technical University, Department of Geological Engineering Ankara ÖZ. — Seyitgazi-Eskişehir antiklinoriumu'nun çok fazla deforme olmuş ve metamorfizmaya uğramış kayaçlarının kuzey-kuzeybatısında bulunan sedimanter ve volkanik kayaçlar incelenmiştir. Karasal ve gölsel fasiyesde meydana gelen Tersi- yer sedimanter kayaçlar Güney Eskişehir küvetinde olunmuşlardır. Karasal fasiye- si meydana getiren kayaç birimlerini kaba kumtaşları, kumtaşları, bitki kalıntıları ihtiva eden kil ve marnlar ve serpantinit blokları taşıyan bazal konglomerası teşkil etmektedir. Gölsel fasiyes ise genellikle killi ve tüflü kalkerler, kalkerler, marnlar, kon- glomeralar ve tüflerden meydana gelmiştir. Küvetteki en eski sedimanlar ve piroklas- tikler Alt Miosen'de oluşmuşlardır. Yataya yakın konumlanmış bazik-intermediyar lav akıntıları Pliosen yaşlı olup Altüst Neojen sedimantasyon kesikliğinde meyda- na gelmiştir. Üst Neojen sedimanter kayaçları intermediyar-basaltik volkaniklerin üzerinde ince bandlar şeklinde bulunurlar. Bu birim marn ve kalkerlerden meyda- na gelmiştir. Alt Miosen’de asid volkanik faaliyetler neticesinde meydana gelen sil- lar (unweldd tuffs) oligomikt konglomeralardan evvel teşekkül etmiştir. Bu volkanik aktitivite muhtemelen kesikli ve kısıtlı olarak devam etmiş ve tüflü kalkerleri mey- dana getirmiştir. Pliosen yaşlı bazik-intermediyar -

Nonexplosive and Explosive Magma/Wet-Sediment Interaction
Journal of Volcanology and Geothermal Research 181 (2009) 155–172 Contents lists available at ScienceDirect Journal of Volcanology and Geothermal Research journal homepage: www.elsevier.com/locate/jvolgeores Nonexplosive and explosive magma/wet-sediment interaction during emplacement of Eocene intrusions into Cretaceous to Eocene strata, Trans-Pecos igneous province, West Texas Kenneth S. Befus a,⁎, Richard E. Hanson a, Daniel P. Miggins b, John A. Breyer a, Arthur B. Busbey a a Department of Geology, Texas Christian University, Box 298830, Fort Worth, TX 76129, USA b U.S. Geological Survey, Denver Federal Center, Box 25046, Denver, CO 80225, USA article info abstract Article history: Eocene intrusion of alkaline basaltic to trachyandesitic magmas into unlithified, Upper Cretaceous Received 16 June 2008 (Maastrichtian) to Eocene fluvial strata in part of the Trans-Pecos igneous province in West Texas produced Accepted 22 December 2008 an array of features recording both nonexplosive and explosive magma/wet-sediment interaction. Intrusive Available online 13 January 2009 complexes with 40Ar/39Ar dates of ~47–46 Ma consist of coherent basalt, peperite, and disrupted sediment. Two of the complexes cutting Cretaceous strata contain masses of conglomerate derived from Eocene fluvial Keywords: deposits that, at the onset of intrusive activity, would have been N400–500 m above the present level of phreatomagmatism peperite exposure. These intrusive complexes are inferred to be remnants of diatremes that fed maar volcanoes during diatreme an early stage of magmatism in this part of the Trans-Pecos province. Disrupted Cretaceous strata along Trans-Pecos Texas diatreme margins record collapse of conduit walls during and after subsurface phreatomagmatic explosions. -

Alkalic-Type Epithermal Gold Deposit Model
Alkalic-Type Epithermal Gold Deposit Model Chapter R of Mineral Deposit Models for Resource Assessment Scientific Investigations Report 2010–5070–R U.S. Department of the Interior U.S. Geological Survey Cover. Photographs of alkalic-type epithermal gold deposits and ores. Upper left: Cripple Creek, Colorado—One of the largest alkalic-type epithermal gold deposits in the world showing the Cresson open pit looking southwest. Note the green funnel-shaped area along the pit wall is lamprophyre of the Cresson Pipe, a common alkaline rock type in these deposits. The Cresson Pipe was mined by historic underground methods and produced some of the richest ores in the district. The holes that are visible along several benches in the pit (bottom portion of photograph) are historic underground mine levels. (Photograph by Karen Kelley, USGS, April, 2002). Upper right: High-grade gold ore from the Porgera deposit in Papua New Guinea showing native gold intergrown with gold-silver telluride minerals (silvery) and pyrite. (Photograph by Jeremy Richards, University of Alberta, Canada, 2013, used with permission). Lower left: Mayflower Mine, Montana—High-grade hessite, petzite, benleonardite, and coloradoite in limestone. (Photograph by Paul Spry, Iowa State University, 1995, used with permission). Lower right: View of north rim of Navilawa Caldera, which hosts the Banana Creek prospect, Fiji, from the portal of the Tuvatu prospect. (Photograph by Paul Spry, Iowa State University, 2007, used with permission). Alkalic-Type Epithermal Gold Deposit Model By Karen D. Kelley, Paul G. Spry, Virginia T. McLemore, David L. Fey, and Eric D. Anderson Chapter R of Mineral Deposit Models for Resource Assessment Scientific Investigations Report 2010–5070–R U.S. -

THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution
VOLCANISM IN HAWAII Chapter 1 - .-............,. THE HAWAIIAN-EMPEROR VOLCANIC CHAIN Part I Geologic Evolution By David A. Clague and G. Brent Dalrymple ABSTRACT chain, the near-fixity of the hot spot, the chemistry and timing of The Hawaiian-Emperor volcanic chain stretches nearly the eruptions from individual volcanoes, and the detailed geom 6,000 km across the North Pacific Ocean and consists of at least etry of volcanism. None of the geophysical hypotheses pro t 07 individual volcanoes with a total volume of about 1 million posed to date are fully satisfactory. However, the existence of km3• The chain is age progressive with still-active volcanoes at the Hawaiian ewell suggests that hot spots are indeed hot. In the southeast end and 80-75-Ma volcanoes at the northwest addition, both geophysical and geochemical hypotheses suggest end. The bend between the Hawaiian and .Emperor Chains that primitive undegassed mantle material ascends beneath reflects a major change in Pacific plate motion at 43.1 ± 1.4 Ma Hawaii. Petrologic models suggest that this primitive material and probably was caused by collision of the Indian subcontinent reacts with the ocean lithosphere to produce the compositional into Eurasia and the resulting reorganization of oceanic spread range of Hawaiian lava. ing centers and initiation of subduction zones in the western Pacific. The volcanoes of the chain were erupted onto the floor of the Pacific Ocean without regard for the age or preexisting INTRODUCTION structure of the ocean crust. Hawaiian volcanoes erupt lava of distinct chemical com The Hawaiian Islands; the seamounts, hanks, and islands of positions during four major stages in their evolution and the Hawaiian Ridge; and the chain of Emperor Seamounts form an growth. -

A Submarine Perspective of the Honolulu Volcanics, Oahu
Journal of Volcanology and Geothermal Research 151 (2006) 279–307 www.elsevier.com/locate/jvolgeores A submarine perspective of the Honolulu Volcanics, Oahu David A. Clague a,*, Jennifer B. Paduan a, William C. McIntosh b, Brian L. Cousens c, Alice´ S. Davis a, Jennifer R. Reynolds d a Monterey Bay Aquarium Research Institute, 7700 Sandholdt Road, Moss Landing, CA 95039-9644, USA b New Mexico Geochronology Research Laboratory, N.M. Bureau of Geology, New Mexico Tech, 801 Leroy Place, Socorro, 87801-4796, USA c Ottawa-Carleton Geoscience Centre, Department of Earth Sciences, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario, Canada K1S 5B6 d School of Fisheries and Ocean Sciences, West Coast and Polar Regions Undersea Research Center, University of Alaska Fairbanks, P.O. Box 757220, 213 O’Neill Building, Fairbanks, AK 99775, USA Accepted 15 July 2005 Available online 27 December 2005 Abstract Lavas and volcaniclastic deposits were observed and collected from 4 submarine cones that are part of the Honolulu Volcanics on Oahu, Hawaii. The locations of these and a few additional, but unsampled, vents demonstrate that nearly all the vents are located on or very close to the shoreline of Oahu, with the most distal vent just 12 km offshore. The clastic samples and outcrops range from coarse breccias to cross-bedded ash deposits and show that explosive volcanism at depths between about 350 and 590 m depth played a part in forming these volcanic cones. The eruptive styles appear to be dominantly effusive to strombolian at greater depths, but apparently include violent phreatomagmatic explosive activity at the shallower sites along the submarine southwest extension of the Koko Rift. -

Basalt-Trachybasalt Samples in Gale Crater, Mars
Open Research Online The Open University’s repository of research publications and other research outputs Basalt-trachybasalt samples in Gale Crater, Mars Journal Item How to cite: Edwards, Peter H.; Bridges, John C.; Wiens, Roger; Anderson, Ryan; Dyar, Darby; Fisk, Martin; Thompson, Lucy; Gasda, Patrick; Filiberto, Justin; Schwenzer, Susanne P.; Blaney, Diana and Hutchinson, Ian (2017). Basalt- trachybasalt samples in Gale Crater, Mars. Meteoritics & Planetary Science, 52(11) pp. 2391–2410. For guidance on citations see FAQs. c 2017 The Authors Meteoritics Planetary Science https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://dx.doi.org/doi:10.1111/maps.12953 Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk Meteoritics & Planetary Science 52, Nr 11, 2391–2410 (2017) doi: 10.1111/maps.12953 Basalt–trachybasalt samples in Gale Crater, Mars Peter H. EDWARDS1, John C. BRIDGES 1*, Roger WIENS2, Ryan ANDERSON3, Darby DYAR4, Martin FISK5, Lucy THOMPSON 6, Patrick GASDA2, Justin FILIBERTO7, Susanne P. SCHWENZER8, Diana BLANEY9, and Ian HUTCHINSON1 1Department of Physics and Astronomy, Leicester Institute for Space and Earth Observation, University of Leicester, Leicester LE1 7RH, UK 2Los Alamos National Lab, Los Alamos, New Mexico 87545, USA 3USGS Astrogeology Science -
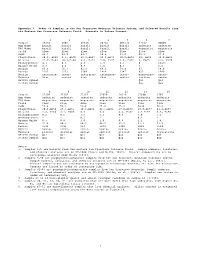
1 Appendix 3. Modes of Samples in the San Francisco Mountain
Appendix 3. Modes of Samples in the San Francisco Mountain Volcanic System, and Selected Basalts from the Eastern San Francisco Volcanic Field. Minerals in Volume Percent. 1 2 3 4 5 6 7 Sample 3823A 2A06 2812A 2031A 2031.B 3732J DC04B Map Name basalt basalt basalt basalt basalt andesite andesite TAS Name basalt basalt basalt basalt basalt mugearite mugearite Field flow flow flow flow flow flow flow SiO2 48.7 48.9 49.7 50.4 51.0 52.2 53.0 Plagioclase 14.6, An69 8.3, An70 25.6,An67 25.5,An71 20.2,An74 29, An64 32.0,An62 Olivine 11.2, Fo83 10.9,Fo84 8.1, Fo73 7.0, Fo77 3.9, Fo76 3, Fo75 6.6, Fo75 Clinopyroxene 4.3 4.3 4.6 1.8 1.3 0 trace Opaque Oxide 4.1 0.2 0.5 1.6 0.3 1 2.4 Matrix 65.8 76.3 61.2 64.1 74.4 67 59.0 Points 1165 1173 1504 1464 1347 827 1549 Matrix intergran- inter- intergran- intergran- inter- microcrys- inter- Texture ular sertal ular ular sertal talline sertal MF1959 Symbol Qa1 Qa1 I-1663 Symbol Qao Qao 8 9 10 11 12 13 14 Sample 3732K 3732Z 3732Y 2705D 3831A 3729Q 3707 Map Name andesite andesite andesite andesite andesite andesite andesite TAS Name mugearite mugearite mugearite mugearite mugearite mugearite mugearite Field flow flow dike flow flow flow flow SiO2 53.4 53.7 53.8 55.2 55.3 56.0 56.6 Plagioclase 24.8,An64 15.3,An61 30.2,An61 42.1,An52 27.0,An62 29.8,An57 41.2,An57 Olivine 2.8, Fo69 3.1, Fo65 4.1 5.6 4.3, Fo62 3.7 Fo57 2.5, Fo58 Clinopyroxene 0.2 0.5 1.5 2.1 0.8 3.0 1.1 Orthopyroxene - - 0.5 - - 0.2 - Opaque Oxide 0.3 0.8 3.0 1.0 0.6 2.1 1.4 Matrix 71.8 80.3 60.7 49.2 67.2 61.1 53.8 Points 1196 1785 854 1844 1428 1029 1540 Matrix hyalo- inter- inter- hyalo- hyalo- hyalo- micro- Texture pilitic granular sertal ophitic pilitic ophitic crystalline MF1959 Symbol Qa1 Qa1 Qai Qa1 Qa2 Qa2 Qa2 I-1663 Symbol Qao Qao Qai Qao Qay Qay Qay Notes: 1. -

Chemical Properties of the Nepheline Basanite from Deposit Husiná
1. Katarína HAKULINOVÁ, 2. Katarína KYSEĽOVÁ, 3. Jana MATULOVÁ A STUDY OF PHYSICO – CHEMICAL PROPERTIES OF THE NEPHELINE BASANITE FROM DEPOSIT HUSINÁ 1‐3. DEPARTMENT OF CHEMISTRY, FACULTY OF METALLURGY, TECHNICAL UNIVERSITY OF KOŠICE, LETNÁ 9, 042 00 KOŠICE, SLOVAKIA ABSTRACT: The submitted article deals with experimental study of chemical and physico‐chemical properties of the nepheline basanite from deposit Husina. The aim of presented work was to study his chemical and mineral composition and melting temperature. The melting temperature measuring was realized using Marsh furnace and high‐temperature microscope. On the base of these basanite properties is possible to appreciate his further industrial utilization. KEYWORDS: nepheline basanite, chemical and mineral composition, melting temperature, thermal analyses INTRODUCTION As approximately 250 stone quarries (in mining, occasionally mining, as a abandoned) mostly based on andesites (presenting the most often exploited rock) are located in Slovakia, vulcanic rock represent one of the most important raw materials needed to produce various forms of the building stone. Andesites and basalt rocks are centrobaric raw materials used for manufacturing of offhand worked stonecutter's products and hammer‐milled gravel aggregate. To its resistance of constant load, resistance of salts and chemical defreezing resources, it is used for roads, paths, squares and other vulnerable places [5]. Basalts in Slovakia have been also mined for petrurgic purposes (fusing basalt). Other opportunities of basalts industrial utilization mostly depend on the knowledge of chemical and physico – chemical properties. PETROGRAPHIC CHARACTERISTIC AND UTILIZATION OF NEPHELINE BASANITE FROM DEPOSIT HUSINÁ Basalts in Slovakia exist in neogene vulcanites mostly in south Slovakia in the surrounding of the Fiľakovo and the Cerová vrchovina Mts. -

THE PETROCHEMISTRY of JAKE M: a MARTIAN MUGEARITE. Stolper
44th Lunar and Planetary Science Conference (2013) 1685.pdf THE PETROCHEMISTRY OF JAKE_M: A MARTIAN MUGEARITE. Stolper, E.M.1, Baker, M.B.1, Fisk, M.2, Gellert, R.3, King, P.L.4, McLennan, S.M.5, Minitti, M.6, Newcombe, M.1, Schmidt, M.E. 7, Treiman, A.H.8, and the MSL Science Team. 1Caltech, Pasadena, CA 91125, 2Oregon State Univ., 3Univ. Guelph, 4Res. School Earth Sci., ANU, 5SUNY, Stony Brook, 6Applied Phys. Lab., Johns Hopkins Univ., 7Brock Univ., 8Lunar & Planet. Inst. Introduction: Rock “Jake_M” (JM; named for JPL The surface of JM was not brushed or abraded prior engineer Jake Matijevic) was the first sample analyzed to analysis, so the APXS analyses probably include by the Alpha Particle X-ray Spectrometer (APXS) in- contributions from surface coatings, including adhering strument on MSL [1]. Although it is an isolated frag- dust, and these are the probable source of the S and Cl ment lacking field context, its dark color and apparently in JM. Experience with MER, however, indicates that fine-grained texture suggested it was a relatively homo- the characteristics of rock compositions are typically geneous igneous rock and thus an appropriate sample to not obscured by surface components, and the levels of S initiate the APXS analytical program. We report here and Cl in JM are lower than virtually all unbrushed the preliminary APXS analyses of JM and a plausible analyses from the Spirit rover and lower than many of interpretation of their significance for petrogenesis. the brushed analyses, so the level of surface contamina- Results: Three spots on JM were analyzed with the tion and alteration are likely relatively minor [5]. -

Direct Derivation of Benmoreite to Phonolite
EXPERIMENTAL DERIVATION OF NEPHELINE SYENITE AND PHONOLITE LIQUIDS BY PARTIAL MELTING OF UPPER MANTLE PERIDOTITES Didier LAPORTE, Sarah LAMBART, Pierre SCHIANO, and Luisa OTTOLINI SUPPLEMENTARY MATERIAL 1. Experimental and analytical techniques 1.1 Starting materials. The starting material used to prepare our two fertile mantle compositions was a fresh spinel lherzolite xenolith (Bri3) from Mont Briançon volcano, French Massif Central. Because a fine grain size is critical to attain chemical equilibrium, lherzolite Bri3 was first pulverized in a micronizing mill for 30 min to reduce its grain size to 2-4 µm. The powder was then fired 5 hours at 900°C in a CO2/H2 atmosphere with gas flow rates adjusted to yield an oxygen fugacity between the magnetite-wüstite and the iron-wüstite -15.91 buffers (fO2 = 10 bar). As Bri3 contains only 100 ppm K2O, small amounts of synthetic basalt B2 were added to Bri3 to prepare the fertile mantle compositions MBK (410 ppm K2O) and MBK+ (930 ppm K2O; compositions Bri3, B2, MBK and MBK+ are given in Table 1). Mixtures of Bri3 and B2 were homogenized by grinding in an agate mortar for one hour. The starting material for reversal experiments MBK+13 and MBK+14 was a mixture of 80.16 % MBK+ and 19.84 % phonolite gel +6 (Table 1). Phonolite gel +6 was prepared using the gel method (Luth and Ingamells, 1965), and then fired at the same T and fO2 as lherzolite Bri3. Gel +6 matches well the target composition (that is, the glass in partial melting experiment MBK+6), except that it is ≈ 10 % richer in Na2O.