Use of Stable Isotopes of Carbon, Nitrogen, and Sulfur to Identify Sources of Nitrogen in Surface Waters in the Lower Susquehanna River Basin, Pennsylvania
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to Isotopic Calculations John M
An Introduction to Isotopic Calculations John M. Hayes ([email protected]) Woods Hole Oceanographic Institution, Woods Hole, MA 02543, USA, 30 September 2004 Abstract. These notes provide an introduction to: termed isotope effects. As a result of such effects, the • Methods for the expression of isotopic abundances, natural abundances of the stable isotopes of practically • Isotopic mass balances, and all elements involved in low-temperature geochemical • Isotope effects and their consequences in open and (< 200°C) and biological processes are not precisely con- closed systems. stant. Taking carbon as an example, the range of interest is roughly 0.00998 ≤ 13F ≤ 0.01121. Within that range, Notation. Absolute abundances of isotopes are com- differences as small as 0.00001 can provide information monly reported in terms of atom percent. For example, about the source of the carbon and about processes in 13 13 12 13 atom percent C = [ C/( C + C)]100 (1) which the carbon has participated. A closely related term is the fractional abundance The delta notation. Because the interesting isotopic 13 13 fractional abundance of C ≡ F differences between natural samples usually occur at and 13F = 13C/(12C + 13C) (2) beyond the third significant figure of the isotope ratio, it has become conventional to express isotopic abundances These variables deserve attention because they provide using a differential notation. To provide a concrete the only basis for perfectly accurate mass balances. example, it is far easier to say – and to remember – that Isotope ratios are also measures of the absolute abun- the isotope ratios of samples A and B differ by one part dance of isotopes; they are usually arranged so that the per thousand than to say that sample A has 0.3663 %15N more abundant isotope appears in the denominator and sample B has 0.3659 %15N. -

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements . -

Stable Isotopes in River Ice: Identifying Primary Over-Winter Streamflow
HYDROLOGICAL PROCESSES Hydrol. Process. 16, 873–890 (2002) DOI: 10.1002/hyp.366 Stable isotopes in river ice: identifying primary over-winter streamflow signals and their hydrological significance J. J. Gibson1* and T. D. Prowse2 1 Department of Earth Sciences, University of Waterloo, Waterloo, Ont. N2L 3G1, Canada 2 National Water Research Institute, 11 Innovation Blvd., Saskatoon, Sask. S7N 3H5, Canada Abstract: The process of isotopic fractionation during freezing in the riverine environment is discussed with reference to a multi-year isotope sampling survey conducted in the Liard–Mackenzie River Basins, northwestern Canada. Systematic isotopic patterns are evident in cores of congelation ice (black ice) obtained from rivers and from numerous tributaries that are recognized as primary streamflow signals but with isotope offsets close to the equilibrium ice–water fractionation. The results, including comparisons with the isotopic composition of fall and spring streamflow measured directly in water samples, suggest that isotopic shifts during ice-on occur due to gradual changes in the fraction of flow derived from groundwater, surface water and precipitation sources during the fall to winter recession. Low flow isotopic signatures during ice-on suggest a predominantly groundwater-fed regime during late winter, whereas low flow isotopic signatures during ice-off reflect a mixed groundwater-, surface water- and precipitation-fed regime during late fall. Copyright 2002 John Wiley & Sons, Ltd. KEY WORDS river ice; streamflow; stable isotopes; oxygen-18; deuterium; hydrograph separation; fractionation INTRODUCTION River, lake and sea ice covers are a valuable archive of changing climate, and specifically hydroclimatic conditions occurring through the winter (Ferrick and Prowse, 2000). -

The Dole Effect and Its Variations During the Last 130,000 Years As Measured in the Vostok Ice Core
GLOBAL BIOGEOCHEMICAL CYCLES, VOL. 8, NO. 3, PAGES 363-376, SEPTEMBER 1994 The Dole effect and its variations during the last 130,000 years as measured in the Vostok ice core Michael Bender • and Todd Sowers 2 GraduateSchool of Oceanography,University of RhodeIsland, Kingston Laurent Labeyrie Centredes Faibles Radioactivites, Centre National de la RechercheScientifique, Commissariat a L'EnergieAtomique, Gif-sur- Yvette, France Abstract. We review the currentunderstanding of the Dole effect (the observeddifference between the •5180of atmospheric0 2 andthat of seawater)and its causes, extend the record of variationsin theDole effectback to 130kyr before present using data on the •5180 of 0 2obtained from studying the Vostok ice core(Sowers et al., 1993), anddiscuss the significanceof temporalvariations. The Dole effectreflects oxygenisotope fractionation during photosynthesis, respiration, and hydrologic processes (evaporation, precipitation,and evapotranspiration). Our bestprediction of the present-dayDole effect,+20.8 %0,is considerablylower thanthe observedvalue, + 23.5 %0,and we discusspossible causes of this discrepancy. During the past 130 kyr, the Dole effecthas been 0.05 %0lower thanthe present value, on average.The standarddeviation of the Dole effect from the meanhas been only _+0.2 %0,and the Dole effect is nearly unchangedbetween glacial maxima and interglacial periods. The smallvariability in theDole effect suggeststhat relative rates of primaryproduction in theland and marine realms have been relatively constant.Most periodicvariability in the Dole effectis in the precessionband, suggesting that changes in thisglobal biogeochemical term reflects variations in low-latitudeland hydrology and productivity or possiblyvariability in low-latitudeoceanic productivity. Introduction they can potentially be made to do so. Ice core reconstructionsof the concentrationsof bioactivegases in air Our understanding of the response of the biosphere to provide an integratedglobal signal which complementsthe Pleistoceneclimate change is actually quite limited. -

Chemical and Isotropic Tracers of Natural Gas and Formation Waters in Fractured Shales
Chemical and isotopic tracers of natural gas and formation waters in Note: this document may contain some elements that are not fully fractured shales accessible to users with disabilities. If you need assistance accessing any information in this document, please contact [email protected]. Jennifer McIntosh, Melissa Schlegel, Brittney Bates Department of Hydrology & Water Resources University of Arizona, Tucson AZ EPA Technical Workshop Feb 24-25, 2011 Outline of Presentation 1)) What is the chemical and isotoppgic signature of formation waters and natural gas in fractured shales? 2) How does it compare with shallow drift aquifers, coalbeds, and other deep geologic formations? Illinois Basin-Case Study - 3 organic-rich formations: glacial drift, Penn. coal, & Dev. fractured shale - organicorganic-rich Ordov. ShaleShale, not part ooff this study m 500 20 km Schlegel et al. (in press, 2011, Geochimica et Cosmochimica Acta) Illinois Basin-Case Study - microbial methane in all 3 units - thermogenic methane in shale and coal m 500 20 km Schlegel et al. (in press, 2011, Geochimica et Cosmochimica Acta) Illinois Basin - water & gas samples Shallow aquifers Penn. Coals Dev. Shale Limestone aquifers 9New data (white symbols): Schlegel et al. (in press) 9Previous data (black symbols): McIntosh et al., 2002; Strąpoć et al., 2007, 2008a,b; Coleman et al, 1988 Fingerprint of natural gases 1000000 Shallow Aquifers 100000 High C1/C2, no C3 Coals 10000 1000 Shale 100 10 Shallow aquifers Penn. Coals Dev. Shale Thermogenic gas 1 -80 -75 -70 -65 -60 -55 -50 -45 -40 13 δ CCH4 (ä PDB) 13 9In general, 3 organic-rich units have different gas compositions (C1/C2+C3) and δ C-CH4 values. -

Environmental Isotope Hydrology Environmental Isotope Hydrology Is a Relatively New Field of Investigation Based on Isotopic Variations Observed in Natural Waters
Environmental Isotope Hydrology Environmental isotope hydrology is a relatively new field of investigation based on isotopic variations observed in natural waters. These isotopic characteristics have been established over a broad space and time scale. They cannot be controlled by man, but can be observed and interpreted to gain valuable regional information on the origin, turnover and transit time of water in the system which often cannot be obtained by other techniques. The cost of such investigations is usually relatively small in comparison with the cost of classical hydrological studies. The main environmental isotopes of hydrological interest are the stable isotopes deuterium (hydrogen-2), carbon-13, oxygen-18, and the radioactive isotopes tritium (hydrogen-3) and carbon-14. Isotopes of hydrogen and oxygen are ideal geochemical tracers of water because their concentrations are usually not subject to change by interaction with the aquifer material. On the other hand, carbon compounds in groundwater may interact with the aquifer material, complicating the interpretation of carbon-14 data. A few other environmental isotopes such as 32Si and 2381//234 U have been proposed recently for hydrological purposes but their use has been quite limited until now and they will not be discussed here. Stable Isotopes of Hydrogen and Oxygen in the Hydrological Cycle The variations of the isotopic ratios D/H and 18O/16O in water samples are expressed in terms of per mille deviation (6%o) from the isotope ratios of mean ocean water, which constitutes the reference standard SMOW: 5%o= (^ RSMOW The isotope ratio, R, is measured using a special mass spectrometer. -

F:\Forensic Geology\Ice Man Isotopes.Wpd
NAME 89.215 - FORENSIC GEOLOGY DEMISE OF THE ICE MAN - ISOTOPIC EVIDENCE I. Introduction Stable and radiogenic isotopic data have been used in a variety of fields to answer a wide range of scientific questions. The nucleus of an atom consists of protons (+1 charge) and neutrons (0 charge), two types of particles that have essentially the same atomic mass. The number of protons in a nucleus determines the element. For example, a nucleus with 1 proton is a hydrogen nucleus, a nucleus with 2 protons is a helium nucleus. Isotopes of an element contain the same number of protons but different numbers of neutrons. For example, there are three isotopes of hydrogen: (1) ordinary hydrogen which contains one proton and no neutrons and has an atomic mass of one; (2) deuterium which contains one proton and one neutron and has an atomic mass of two; and (3) tritium which contains one proton and two neutrons and has an atomic mass of three. The convention used to show the numbers of types of particles in a nucleus is to place the number of protons (called the atomic number) at the bottom left of the element symbol and the number of protons plus neutrons (called the atomic mass) at the upper left of the element symbol. For example, the tritium isotope is 3 shown as 1H . II. Stable isotopes Stable isotopes are not radioactive, they do not spontaneously breakdown to other atoms. In a previous exercise you used radioactive carbon to determine when the Ice Man was killed. There are three isotopes of carbon: (1) carbon 12 which contains 6 protons and 6 neutrons giving an atomic mass of 12; (2) carbon 13 which contains 6 protons and 7 neutrons giving an atomic mass of 13; and (3) carbon 14 which contains 6 protons and 8 neutrons giving an atomic mass of 14. -

Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T
U.S. Department of the Interior U.S. Geological Survey Compilation of Minimum and Maximum Isotope Ratios of Selected Elements in Naturally Occurring Terrestrial Materials and Reagents by T. B. Coplen', J. A. Hopple', J. K. Bohlke', H. S. Reiser', S. E. Rieder', H. R. o *< A R 1 fi Krouse , K. J. R. Rosman , T. Ding , R. D. Vocke, Jr., K. M. Revesz, A. Lamberty, P. Taylor, and P. De Bievre6 'U.S. Geological Survey, 431 National Center, Reston, Virginia 20192, USA 2The University of Calgary, Calgary, Alberta T2N 1N4, Canada 3Curtin University of Technology, Perth, Western Australia, 6001, Australia Institute of Mineral Deposits, Chinese Academy of Geological Sciences, Beijing, 100037, China National Institute of Standards and Technology, 100 Bureau Drive, Stop 8391, Gaithersburg, Maryland 20899 "institute for Reference Materials and Measurements, Commission of the European Communities Joint Research Centre, B-2440 Geel, Belgium Water-Resources Investigations Report 01-4222 Reston, Virginia 2002 U.S. Department of the Interior GALE A. NORTON, Secretary U.S. Geological Survey Charles G. Groat, Director The use of trade, brand, or product names in this report is for identification purposes only and does not imply endorsement by the U.S. Government. For additional information contact: Chief, Isotope Fractionation Project U.S. Geological Survey Mail Stop 431 - National Center Reston, Virginia 20192 Copies of this report can be purchased from: U.S. Geological Survey Branch of Information Services Box 25286 Denver, CO 80225-0286 CONTENTS Abstract...................................................... -
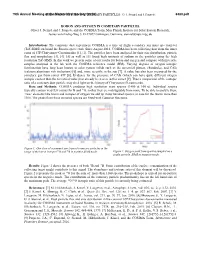
Boron and Oxygen in Cometary Particles: O. J
79th Annual Meeting ofBORON the Meteoritical AND OXYGEN Society IN COMETARY (2016) PARTICLES: O. J. Stenzel and J. Paquette 6380.pdf BORON AND OXYGEN IN COMETARY PARTICLES. Oliver J. Stenzel and J. Paquette and the COSIMA Team, Max Planck Institute for Solar System Research, Justus-von-Liebig-Weg 3, D-37077 Göttingen, Germany, [email protected]. Introduction: The cometary dust experiment COSIMA is a time of flight secondary ion mass spectrometer (ToF-SIMS) on board the Rosetta space craft. Since August 2014, COSIMA has been collecting dust from the inner coma of 67P/Churyumov-Gerasimenko [1], [2]. The particles have been analyzed for their size distribution, particle flux and morphology [3], [4]. [5] as well as [1] found high amounts of sodium in the particles using the high resolution ToF-SIMS. In this work we present some of our results for boron and oxygen and compare with meteorite samples analyzed in the lab with the COSIMA reference model (RM). Varying degrees of oxygen isotopic fractionation have long been known in solar system solids such as the terrestrial planets, chondrules, and CAIs (calcium-aluminum-rich inclusions) [6] and, more recently, in the sun [7]. A value has also been measured for the cometary gas from comet 67P [8]. Evidence for the presence of CAIs (which can have quite different oxygen isotopic content than the terrestrial value) has already been seen in this comet [9]. Thus a comparison of the isotopic ratio of a cometary dust particle may shed light on the history of Churyumov-Gerasimenko. Data and Methods: COSIMA produces high resolution mass spectra (1400 at 100 u). -

Investigation of Nitrogen Cycling Using Stable Nitrogen and Oxygen Isotopes in Narragansett Bay, Ri
University of Rhode Island DigitalCommons@URI Open Access Dissertations 2014 INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI Courtney Elizabeth Schmidt University of Rhode Island, [email protected] Follow this and additional works at: https://digitalcommons.uri.edu/oa_diss Recommended Citation Schmidt, Courtney Elizabeth, "INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI" (2014). Open Access Dissertations. Paper 209. https://digitalcommons.uri.edu/oa_diss/209 This Dissertation is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Dissertations by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. INVESTIGATION OF NITROGEN CYCLING USING STABLE NITROGEN AND OXYGEN ISOTOPES IN NARRAGANSETT BAY, RI BY COURTNEY ELIZABETH SCHMIDT A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN OCEANOGRAPHY UNIVERSITY OF RHODE ISLAND 2014 DOCTOR OF PHILOSOPHY DISSERTATION OF COURTNEY ELIZABETH SCHMIDT APPROVED: Thesis Committee: Major Professor Rebecca Robinson Candace Oviatt Arthur Gold Nassar H. Zawia DEAN OF THE GRADUATE SCHOOL UNIVERSITY OF RHODE ISLAND 2014 ABSTRACT Estuaries regulate nitrogen (N) fluxes transported from land to the open ocean through uptake and denitrification. In Narragansett Bay, anthropogenic N loading has increased over the last century with evidence for eutrophication in some regions of Narragansett Bay. Increased concerns over eutrophication prompted upgrades at wastewater treatment facilities (WWTFs) to decrease the amount of nitrogen discharged. The upgrade to tertiary treatment – where bioavailable nitrogen is reduced and removed through denitrification – has occurred at multiple facilities throughout Narragansett Bay’s watershed. -

Analytical Developments in the Measurements of Boron, Nitrate
XA9848324 ANALYTICAL DEVELOPMENTS IN THE MEASUREMENTS OF BORON, NITRATE, PHOSPHATE AND SULPHATE ISOTOPES AND CASE EXAMPLES OF DISCRIMINATION OF NITROGEN AND SULPHUR SOURCES IN POLLUTION STUDIES J. AGGARWAL, D.S. SHEPPARD Institute of Geological & Nuclear Sciences, Lower Hutt B.W. ROBINSON Wairakei Research Centre, Institute of Geological & Nuclear Sciences, Taupo New Zealand Abstract - Methods are documented for the analysis of B isotopes, O and N isotopes in nitrates. B isotopes can be measured by negative ion thermal ionisation mass spectrometry. Nitrate is recovered from groundwaters by ion exchange and the resulting silver nitrate combusted for stable isotope gas analysis. Oxygen isotope analysis of phosphates can be determined by generating and analysing CO2 gas from the combustion of silver phosphate produced from aqueous samples. Sulphate in ground and surface waters can be separated and concentrated by ion exchange and precipitated as barium sulphate. This is reacted with graphite to yield CO2 and CO, the latter being spark discharged to CO2 and the total CO2 measured for oxygen isotope analysis. Barium sulphide from this reaction is converted to silver sulphide which is reacted with cuprous oxide to give SO2 gas for sulphur isotope measurements. A case study of the semi-rural Manakau area in New Zealand was conducted to see if nitrate isotopes could be used to detect the source of nitrate contamination (groundwater nitrate <0.5 to 11.3 mg/L NO'3-N). Nitrogen isotope (+4 to +12%o) coupled with oxygen isotope measurements (+5 to +9%o) demonstrated that the nitrogen is not sourced from fertilisers but from some combination of septic tank and animal waste. -

Environmental Isotopes in Hydrogeology
Environmental Isotopes in Hydrogeology IanD. Clark and Peter Fritz www.science.uottawa.ca/~eih CRC Press Taylor & Francis Group Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an inform a business Library of Congress Cataloging-in-Publication Data Clark, Ian D. (Ian Douglas), 1954- Environmental Isotopes in Hydrogeology / Ian D, Clark and Peter Fritz p. cm. Includes bibliographical references and index. ISBN 1-56670-249-6 (alk. paper) 1. Radioactive tracers in hydrogeology. I. Fritz, P. (Peter), 1937- II. Title. GB1001.72.R34C57 551.49'028—dc21 97-21889 CIP This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. Neither this book nor any part may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, microfilming, and recording, or by any information storage or retrieval system, without prior permission in writing from the publisher. The consent of CRC Press LLC does not extend to copying for general distribution, for promotion, for creating new works, or for resale. Specific permission must be obtained in writing from CRC Press LLC for such copying. Trademark Notice: Product or corporate names may be trademarks or registered trademarks, and are used only for identification and explanation, without intent to infringe.