Isotope Geochemistry of Carbonate Dissolution and Reprecipitation in Soils R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

THE NATURAL RADIOACTIVITY of the BIOSPHERE (Prirodnaya Radioaktivnost' Iosfery)
XA04N2887 INIS-XA-N--259 L.A. Pertsov TRANSLATED FROM RUSSIAN Published for the U.S. Atomic Energy Commission and the National Science Foundation, Washington, D.C. by the Israel Program for Scientific Translations L. A. PERTSOV THE NATURAL RADIOACTIVITY OF THE BIOSPHERE (Prirodnaya Radioaktivnost' iosfery) Atomizdat NMoskva 1964 Translated from Russian Israel Program for Scientific Translations Jerusalem 1967 18 02 AEC-tr- 6714 Published Pursuant to an Agreement with THE U. S. ATOMIC ENERGY COMMISSION and THE NATIONAL SCIENCE FOUNDATION, WASHINGTON, D. C. Copyright (D 1967 Israel Program for scientific Translations Ltd. IPST Cat. No. 1802 Translated and Edited by IPST Staff Printed in Jerusalem by S. Monison Available from the U.S. DEPARTMENT OF COMMERCE Clearinghouse for Federal Scientific and Technical Information Springfield, Va. 22151 VI/ Table of Contents Introduction .1..................... Bibliography ...................................... 5 Chapter 1. GENESIS OF THE NATURAL RADIOACTIVITY OF THE BIOSPHERE ......................... 6 § Some historical problems...................... 6 § 2. Formation of natural radioactive isotopes of the earth ..... 7 §3. Radioactive isotope creation by cosmic radiation. ....... 11 §4. Distribution of radioactive isotopes in the earth ........ 12 § 5. The spread of radioactive isotopes over the earth's surface. ................................. 16 § 6. The cycle of natural radioactive isotopes in the biosphere. ................................ 18 Bibliography ................ .................. 22 Chapter 2. PHYSICAL AND BIOCHEMICAL PROPERTIES OF NATURAL RADIOACTIVE ISOTOPES. ........... 24 § 1. The contribution of individual radioactive isotopes to the total radioactivity of the biosphere. ............... 24 § 2. Properties of radioactive isotopes not belonging to radio- active families . ............ I............ 27 § 3. Properties of radioactive isotopes of the radioactive families. ................................ 38 § 4. Properties of radioactive isotopes of rare-earth elements . -

Chemical and Isotropic Tracers of Natural Gas and Formation Waters in Fractured Shales
Chemical and isotopic tracers of natural gas and formation waters in Note: this document may contain some elements that are not fully fractured shales accessible to users with disabilities. If you need assistance accessing any information in this document, please contact [email protected]. Jennifer McIntosh, Melissa Schlegel, Brittney Bates Department of Hydrology & Water Resources University of Arizona, Tucson AZ EPA Technical Workshop Feb 24-25, 2011 Outline of Presentation 1)) What is the chemical and isotoppgic signature of formation waters and natural gas in fractured shales? 2) How does it compare with shallow drift aquifers, coalbeds, and other deep geologic formations? Illinois Basin-Case Study - 3 organic-rich formations: glacial drift, Penn. coal, & Dev. fractured shale - organicorganic-rich Ordov. ShaleShale, not part ooff this study m 500 20 km Schlegel et al. (in press, 2011, Geochimica et Cosmochimica Acta) Illinois Basin-Case Study - microbial methane in all 3 units - thermogenic methane in shale and coal m 500 20 km Schlegel et al. (in press, 2011, Geochimica et Cosmochimica Acta) Illinois Basin - water & gas samples Shallow aquifers Penn. Coals Dev. Shale Limestone aquifers 9New data (white symbols): Schlegel et al. (in press) 9Previous data (black symbols): McIntosh et al., 2002; Strąpoć et al., 2007, 2008a,b; Coleman et al, 1988 Fingerprint of natural gases 1000000 Shallow Aquifers 100000 High C1/C2, no C3 Coals 10000 1000 Shale 100 10 Shallow aquifers Penn. Coals Dev. Shale Thermogenic gas 1 -80 -75 -70 -65 -60 -55 -50 -45 -40 13 δ CCH4 (ä PDB) 13 9In general, 3 organic-rich units have different gas compositions (C1/C2+C3) and δ C-CH4 values. -

F:\Forensic Geology\Ice Man Isotopes.Wpd
NAME 89.215 - FORENSIC GEOLOGY DEMISE OF THE ICE MAN - ISOTOPIC EVIDENCE I. Introduction Stable and radiogenic isotopic data have been used in a variety of fields to answer a wide range of scientific questions. The nucleus of an atom consists of protons (+1 charge) and neutrons (0 charge), two types of particles that have essentially the same atomic mass. The number of protons in a nucleus determines the element. For example, a nucleus with 1 proton is a hydrogen nucleus, a nucleus with 2 protons is a helium nucleus. Isotopes of an element contain the same number of protons but different numbers of neutrons. For example, there are three isotopes of hydrogen: (1) ordinary hydrogen which contains one proton and no neutrons and has an atomic mass of one; (2) deuterium which contains one proton and one neutron and has an atomic mass of two; and (3) tritium which contains one proton and two neutrons and has an atomic mass of three. The convention used to show the numbers of types of particles in a nucleus is to place the number of protons (called the atomic number) at the bottom left of the element symbol and the number of protons plus neutrons (called the atomic mass) at the upper left of the element symbol. For example, the tritium isotope is 3 shown as 1H . II. Stable isotopes Stable isotopes are not radioactive, they do not spontaneously breakdown to other atoms. In a previous exercise you used radioactive carbon to determine when the Ice Man was killed. There are three isotopes of carbon: (1) carbon 12 which contains 6 protons and 6 neutrons giving an atomic mass of 12; (2) carbon 13 which contains 6 protons and 7 neutrons giving an atomic mass of 13; and (3) carbon 14 which contains 6 protons and 8 neutrons giving an atomic mass of 14. -

Mass Fraction and the Isotopic Anomalies of Xenon and Krypton in Ordinary Chondrites
Scholars' Mine Masters Theses Student Theses and Dissertations 1971 Mass fraction and the isotopic anomalies of xenon and krypton in ordinary chondrites Edward W. Hennecke Follow this and additional works at: https://scholarsmine.mst.edu/masters_theses Part of the Chemistry Commons Department: Recommended Citation Hennecke, Edward W., "Mass fraction and the isotopic anomalies of xenon and krypton in ordinary chondrites" (1971). Masters Theses. 5453. https://scholarsmine.mst.edu/masters_theses/5453 This thesis is brought to you by Scholars' Mine, a service of the Missouri S&T Library and Learning Resources. This work is protected by U. S. Copyright Law. Unauthorized use including reproduction for redistribution requires the permission of the copyright holder. For more information, please contact [email protected]. MASS FRACTIONATION AND THE ISOTOPIC ANOMALIES OF XENON AND KRYPTON IN ORDINARY CHONDRITES BY EDWARD WILLIAM HENNECKE, 1945- A THESIS Presented to the Faculty of the Graduate School of the UNIVERSITY OF MISSOURI-ROLLA In Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE IN CHEMISTRY 1971 T2572 51 pages by Approved ~ (!.{ 1.94250 ii ABSTRACT The abundance and isotopic composition of all noble gases are reported in the Wellman chondrite, and the abundance and isotopic composition of xenon and krypton are reported in the gases released by stepwise heating of the Tell and Scurry chondrites. Major changes in the isotopic composition of xenon result from the presence of radio genic Xel29 and from isotopic mass fractionation. The isotopic com position of trapped krypton in the different temperature fractions of the Tell and Scurry chondrites also shows the effect of isotopic fractiona tion, and there is a covariance in the isotopic composition of xenon with krypton in the manner expected from mass dependent fractiona tion. -

Nuclear and Isotopic Techniques for Marine Pollution Monitoring
NUCLEAR AND ISOTOPIC TECHNIQUES FOR MARINE POLLUTION MONITORING A. Introduction The greatest natural resource on the Planet, the World Ocean is both the origin of most life forms and the source of survival for hundredsth of millions of people. Pollution of the oceans was an essential problem of the 20 century that was associated with the rapid industrializationst and unplanned occupation of coastal zones It continues to be a concern in the 21 century. The most serious environmental problems encountered in coastal zones are presented by runoff of agricultural nutrients, heavy metals, and persistent organic pollutants, such as pesticides and plastics. Oil spills from ships and tankers continually present a serious threat to birds, marine life and beaches. Also, many pesticides that are banned in most industrialized countries remain in use today, their trans-boundary pathway of dispersion affecting marine ecosystems the world over. In addition to these problems, ocean dumping of radioactive waste has occurred in the past, and might occur again, with authorised discharges of radioactive substances from nuclear facilities into rivers and coastal areas contributing to the contamination of the marine environment. A trans-boundary issue, marine pollution is often caused by inland economic activities, which lead to ecological changes in the marine environment. Examples include the use of chemicals in agriculture, atmospheric emissions from factories and automobiles, sewage discharges into lakes and rivers, and many other phenomena taking place hundreds or thousands of kilometres away from the seashore. Sooner or later, these activities affect the ecology of estuaries, bays, coastal waters, and sometimes entire seas, consequently having an impact on the economy related to maritime activities. -

Stable Isotopes of Carbon and Nitrogen in the Study of Avian and Mammalian Trophic Ecology
Color profile: Disabled Composite Default screen 1 REVIEW/SYNTHÈSE Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology Jeffrey F. Kelly Abstract: Differential fractionation of stable isotopes of carbon during photosynthesis causes C4 plants and C3 plants to have distinct carbon-isotope signatures. In addition, marine C3 plants have stable-isotope ratios of carbon that are 13 12 intermediate between C4 and terrestrial C3 plants. The direct incorporation of the carbon-isotope ratio ( C/ C) of plants into consumers’ tissues makes this ratio useful in studies of animal ecology. The heavy isotope of nitrogen (15N) is preferentially incorporated into the tissues of the consumer from the diet, which results in a systematic enrichment in nitrogen-isotope ratio (15N/14N) with each trophic level. Consequently, stable isotopes of nitrogen have been used pri- marily to assess position in food chains. The literature pertaining to the use of stable isotopes of carbon and nitrogen in animal trophic ecology was reviewed. Data from 102 studies that reported stable-isotope ratios of carbon and (or) nitrogen of wild birds and (or) mammals were compiled and analyzed relative to diet, latitude, body size, and habitat moisture. These analyses supported the predicted relationships among trophic groups. Carbon-isotope ratios differed among species that relied on C3,C4, and marine food chains. Likewise, nitrogen-isotope ratios were enriched in terres- trial carnivorous mammals relative to terrestrial herbivorous mammals. -

Correcting for Naturally Occurring Mass Isotopologue Abundances in Stable-Isotope Tracing Experiments with Polymid
H OH metabolites OH Article Correcting for Naturally Occurring Mass Isotopologue Abundances in Stable-Isotope Tracing Experiments with PolyMID Heesoo Jeong 1, Yan Yu 2, Henrik J. Johansson 3, Frank C. Schroeder 2, Janne Lehtiö 3 and Nathaniel M. Vacanti 1,3,* 1 Division of Nutritional Sciences, Cornell University, Ithaca, NY 14853, USA; [email protected] 2 Boyce Thompson Institute and Department of Chemistry and Chemical Biology, Cornell University, Ithaca, NY 14853, USA; [email protected] (Y.Y.); [email protected] (F.C.S.) 3 Science for Life Laboratory, Department of Oncology-Pathology, Karolinska Institutet, 17165 Solna, Sweden; [email protected] (H.J.J.); [email protected] (J.L.) * Correspondence: [email protected] Abstract: Stable-isotope tracing is a method to measure intracellular metabolic pathway utilization by feeding a cellular system a stable-isotope-labeled tracer nutrient. The power of the method to resolve differential pathway utilization is derived from the enrichment of metabolites in heavy isotopes that are synthesized from the tracer nutrient. However, the readout is complicated by the presence of naturally occurring heavy isotopes that are not derived from the tracer nutrient. Herein we present an algorithm, and a tool that applies it (PolyMID-Correct, part of the PolyMID software package), to computationally remove the influence of naturally occurring heavy isotopes. Citation: Jeong, H.; Yu, Y.; Johansson, The algorithm is applicable to stable-isotope tracing data collected on low- and high- mass resolution H.J.; Schroeder, F.C.; Lehtiö, J.; mass spectrometers. PolyMID-Correct is open source and available under an MIT license. Vacanti, N.M. -
How Atoms Differ A
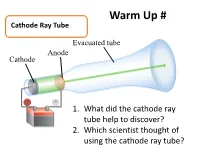
Warm Up # Cathode Ray Tube Evacuated tube Anode Cathode − + 1. What did the cathode ray tube help to discover? Battery 2. Which scientist thought of using the cathode ray tube? How Atoms Differ a. Properties of Subatomic Particles Particle Symbol Location Relative Relative Actual Charge mass mass (g) outside 1 Electron e- the 9.11 x nucleus -1 1840 10-28 g in the 1.673 x Proton p+ nucleus +1 1 10-24 g in the 1.675 x Neutron 0 n nucleus 0 1 10-24 g Elements on the Periodic Table b. Atomic Number • the number of protons in an atom • Identifies element c. Mass Number •represents the total number of protons and neutrons in the nucleus Mass number A ZX atomic number d. Isotopes • Atoms that have the same number of protons but have a different masses • Ex: 3 isotopes of carbon: 12 13 14 6C 6C 6C e. Average Atomic Mass • the weighted average of the isotopes of that element. • Formula: % mass % Atomic mass abundance of abundance mass of ( ) ( of ) = of x Isotope + of x + … an element Isotope #2 Isotope #1 #1 Isotope #2 Average Atomic Mass • The mass of an atom is so small it is difficult to work with, so chemists have developed an atomic standard to compare all the masses • The standard is the atomic mass unit (amu) • If the mass of an element is not close to a whole number, it is because the atom has several isotopes • The atomic mass is the weighted average of the isotopes of that element Example 1 Silver has two naturally occurring isotopes. -

Office of Radiation Protection Department of Health 7171 Cleanwater Lane, Bldg
DOH 320-029 Environmental Radiation Program Hanford Environmental Oversight Program 2000 Data Summary Report Environmental Health Programs DOH 320-029 Environmental Radiation Program Hanford Environmental Oversight Program 2000 Data Summary Report Scott Van Verst Lynn Albin Washington State Department of Health For more information or additional copies of this report, contact: Environmental Radiation Section Office of Radiation Protection Department of Health 7171 Cleanwater Lane, Bldg. 5 P.O. Box 47827 Olympia WA 98504 360-236-3251 Email: [email protected] FAX: 360-236-2255 Summary The Washington State Department of Health (DOH) has operated an environmental radiation monitoring program since 1961. The early program looked primarily at atmospheric fallout and off-site environmental impacts related to Hanford operations. Currently, the DOH conducts radiological surveillance in many geographical areas of the state and routinely splits (co-samples) environmental samples with state licensed and federal environmental monitoring programs. Since 1985, the Washington State Department of Health’s Hanford Environmental Oversight Program has participated with the U.S. Department of Energy (DOE) in the collection of environmental samples on or near the Hanford Site. The purpose of the program is to independently verify the quality of DOE environmental monitoring programs at the Hanford Site, and to assess the potential for public health impacts. This report is a summary of the data collected for the Hanford Environmental Oversight Program in 2000. The Oversight Program’s objectives are met through collection and analyses of environmental samples and interpretation of results. DOH samples are either split or co-located with samples collected by the DOE contractors. -

Isotopes Are Atoms with the Same # of Protons (Making Them the SAME ELEMENT), but Have a Different # of Neutrons
Each element has a unique # of protons. So…EVERY SINGLE ATOM of that element MUST HAVE the SAME number of protons, ALWAYS! This is the reason why each element has a unique Atomic Number that never changes! Isotopes are atoms with the same # of protons (making them the SAME ELEMENT), but have a different # of neutrons. Having different # of neutrons for the same element means that the isotopes will have different mass numbers, too! There are 3 isotopes of oxygen: 8 8 8 O8 O9 O10 neutronsOxygen neutronsOxygen neutronsOxygen ALL oxygen16 isotopes 17have an atomic 18# of 8, so they all have 8 protons! How many protons does each isotope have? How many neutrons does each isotope have? There are 3 isotopes of carbon: 6 6 6 C6 C7 C8 neutronsCarbon neutronsCarbon neutronsCarbon ALL carbon12 isotopes 13have an atomic #14 of 6, so they all have 6 protons! How many protons does each isotope have? How many neutrons does each isotope have? Atomic Masses on the periodic table are normally decimals. WHY?? Atomic Masses are decimals because it is an AVERAGE mass of all of the element’s isotopes. Some isotopes are more common than others, so this is a weighted average. 5 So the Mass # from the periodic table is B always closest to the most common Boron isotope’s mass. 10.81 In the universe, there are MUCH more carbon-12 atoms that exist than carbon-13 and carbon-14 atoms. 6 C Carbon- 12 Carbon 12.01 When you find the weighted average of the masses of the carbon isotopes, the average will be closest to 12! The Atomic Mass of calcium is 40.08. -

Isotopic Compositions of the Elements 1983
Pure & Appi. Chem., Vol. 56, No. 6, pp. 675—694, 1984. 0033—4545/84 $3.00+0.00 Printed in Great Britain. Pergamon Press Ltd ©1984 IUPAC INTERNATIONALUNION OF PURE AND APPLIED CHEMISTRY INORGANIC CHEMISTRY DIVISION COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCES* ISOTOPIC COMPOSITIONS OF THE ELEMENTS 1983 Prepared for publication by N. E. HOLDEN1, R. L. MARTIN2 and I. L. BARNES3 1Brookhaven National Laboratory, Upton, New York 11973, USA 2Monash University, Clayton, Victoria 3168, Australia 3National Bureau of Standards, Washington, DC 20234, USA *Membership of the Commission for the period 1981—85 is as follows: R. L. MARTIN (Chairman 1983—85, Secretary 1981—83); J. R. DE LAETER (Secretary 1983—85, Associate 1981—83); R. C. BARBER (Titular 1981—85); I. L. BARNES (Titular 1981—85); P. DE BIEVRE (Associate 1981—85); T. L. CHANG (Titular 1983—85); T.B. COPLEN (Associate 1983—85); R. GONFIANTINI (Associate 1981—83); N. N. GREENWOOD (Associate 1981—83); R. HAGEMANN (Titular 1981—83); K. G. HEUMANN (Associate 1983—85); N. E. HOLDEN (Associate 1983—85, Chairman 1981—83); W. H. JOHNSON (Associate 1981—85); T. J. MURPHY (Titular 1981—85); H. S. PEISER (Associate 1981—85); C. E. REES (Associate 1983—85); K. J. R. ROSMAN (Associate 1983—85); M. SHIMA (Associate 1981—85). ISOTOPIC COMPOSITIONS OF THE ELEMENTS 1983 Abstract —TheCommission's biennial review of isotopic compositions as determined by mass spectrometry and other relevant methods has been undertaken by the Subcommittee for the Assessment of Isotopic Composition (SAIC). The Subcommittee's critical evaluation of the published literature element by element forms the basis of the Table of Isotopic Compositions as Determined by Mass Spectrometry, 1983, which is presented in this Report. -

Calcium Isotope Fractionation and Its Controlling Factors Over Authigenic Carbonates in the Cold Seeps of the Northern South China Sea
Article Oceanology April 2012 Vol.57 No.11: 13251332 doi: 10.1007/s11434-012-4990-9 Calcium isotope fractionation and its controlling factors over authigenic carbonates in the cold seeps of the northern South China Sea WANG ShuHong1, YAN Wen1*, MAGALHÃES H Vitor2, CHEN Zhong1, PINHEIRO M Luis2 & GUSSONE Nikolaus3 1 CAS Key Laboratory of Marginal Sea Geology, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, China; 2 Centre for Environmental and Marine Studies (CESAM) and Geosciences Department, University of Aveiro, Aveiro 3810-193, Portugal; 3 Institut für Mineralogie, Universität Münster, Münster 48149, Germany Received November 7, 2011; accepted December 23, 2011; published online February 21, 2012 In this study, we analyzed stable calcium isotope results of authigenic carbonates from two cold seep areas of the Dongsha area and the Baiyun Sag in the northern South China Sea. The stable isotopes of carbon and oxygen as well as the mineral composition 44/40 of authigenic carbonates were used to investigate control calcium isotope fractionation. The Ca ratios of the southwestern Dongsha area samples ranged from 1.21‰ to 1.52‰ and the ratio of the Baiyun Sag sample was 1.55‰ of the SRM915a isotope standard. X-ray diffraction analysis showed that the carbonate samples consisted of dolomite, calcite and aragonite, with small amounts of high-Mg calcite and siderite. The 13C values of the carbonates of the southwestern Dongsha area varied between 18 49.21‰ and 16.86‰ of the Vienna PeeDee Belemnite (VPDB) standard and the O values ranged from 2.25‰ to 3.72‰ 13 18 13 VPDB.