Liparis Crenulata (Orchidaceae, Liparidinae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Redalyc.Chemical Composition of the Inflorescence Odor of Malaxis
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Kite, Geoffrey C.; Salazar, Gerardo A. Chemical composition of the inflorescence odor of Malaxis rzedowskiana (Orchidaceae) Revista Mexicana de Biodiversidad, vol. 79, núm. 1, 2008, pp. 153-157 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42558786026 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Biodiversidad 79: 153- 157, 2008 Chemical composition of the infl orescence odor of Malaxis rzedowskiana (Orchidaceae) Composición química del olor de la infl orescencia de Malaxis rzedowskiana (Orchidaceae) Geoffrey C. Kite1 and Gerardo A. Salazar2* 1Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3DS, United Kingdom 2Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70-367, 04510 México, D.F., Mexico *Correspondent: [email protected] Abstract. Malaxis rzedowskiana R.González (Malaxideae, Orchidaceae) from Mexico produces a pleasant fl oral odor reminiscent of violets in contrast to the unpleasant odors noted for several other members of Malaxideae. Analysis of the fl oral odor of M. rzedowskiana by headspace trapping and thermal desorption-gas chromatography-mass spectrometry revealed the presence of kaurene (76%), (E)-ß-ionone (18%) and (E)-a-ionone (4%) as the main components. This is the fi rst report of a fl oral odor containing a high proportion of kaurene. -

Ecology of Pyrmont Peninsula 1788 - 2008
Transformations: Ecology of Pyrmont peninsula 1788 - 2008 John Broadbent Transformations: Ecology of Pyrmont peninsula 1788 - 2008 John Broadbent Sydney, 2010. Ecology of Pyrmont peninsula iii Executive summary City Council’s ‘Sustainable Sydney 2030’ initiative ‘is a vision for the sustainable development of the City for the next 20 years and beyond’. It has a largely anthropocentric basis, that is ‘viewing and interpreting everything in terms of human experience and values’(Macquarie Dictionary, 2005). The perspective taken here is that Council’s initiative, vital though it is, should be underpinned by an ecocentric ethic to succeed. This latter was defined by Aldo Leopold in 1949, 60 years ago, as ‘a philosophy that recognizes[sic] that the ecosphere, rather than any individual organism[notably humans] is the source and support of all life and as such advises a holistic and eco-centric approach to government, industry, and individual’(http://dictionary.babylon.com). Some relevant considerations are set out in Part 1: General Introduction. In this report, Pyrmont peninsula - that is the communities of Pyrmont and Ultimo – is considered as a microcosm of the City of Sydney, indeed of urban areas globally. An extensive series of early views of the peninsula are presented to help the reader better visualise this place as it was early in European settlement (Part 2: Early views of Pyrmont peninsula). The physical geography of Pyrmont peninsula has been transformed since European settlement, and Part 3: Physical geography of Pyrmont peninsula describes the geology, soils, topography, shoreline and drainage as they would most likely have appeared to the first Europeans to set foot there. -

Australian Orchid Name Index (16/2/2007)
AUSTRALIAN ORCHID NAME INDEX (16/2/2007) by Mark A. Clements and David L. Jones Centre for Plant Biodiversity Research/Australian National Herbarium GPO Box 1600 Canberra ACT 2601 Australia Corresponding author: [email protected] INTRODUCTION The Australian Orchid Name Index (AONI) provides the currently accepted scientific names, together with their synonyms, of all Australian orchids including those in external territories. The appropriate scientific name for each orchid taxon is based on data published in the scientific or historical literature, and/or from study of the relevant type specimens or illustrations and study of taxa as herbarium specimens, in the field or in the living state. Structure of the index: Genera and species are listed alphabetically. Accepted names for taxa are in bold, followed by the author(s), place and date of publication, details of the type(s), including where it is held and assessment of its status. The institution(s) where type specimen(s) are housed are recorded using the international codes for Herbaria (Appendix 1) as listed in Holmgren et al’s Index Herbariorum (1981) continuously updated, see [http://sciweb.nybg.org/science2/IndexHerbariorum.asp]. Citation of authors follows Brummit & Powell (1992) Authors of Plant Names; for book abbreviations, the standard is Taxonomic Literature, 2nd edn. (Stafleu & Cowan 1976-88; supplements, 1992-2000); and periodicals are abbreviated according to B-P- H/S (Bridson, 1992) [http://www.ipni.org/index.html]. Synonyms are provided with relevant information on place of publication and details of the type(s). They are indented and listed in chronological order under the accepted taxon name. -
Native Orchid Society of South Australia
NATIVE ORCHID SOCIETY of SOUTH AUSTRALIA NATIVE ORCHID SOCIETY OF SOUTH AUSTRALIA JOURNAL Volume 7, No. 3, April, 1983 Registered by Australia Post Publication No. SBH 1344. Price 40c PATRON: Mr T.R.N. Lothian PRESIDENT: Mr J.T. Simmons SECRETARY: Mr E.R. Hargreaves 4 Gothic Avenue 1 Halmon Avenue STONYFELL S.A. 5066 EVERARD PARK SA 5035 Telephone 32 5070 Telephone 293 2471 297 3724 VICE-PRESIDENT: Mr G.J. Nieuwenhoven COMMITTFE: Mr R. Shooter Mr P. Barnes TREASURER: Mr R.T. Robjohns Mrs A. Howe Mr R. Markwick EDITOR: Mr G.J. Nieuwenhoven NEXT MEETING When: Tuesday 26 April, 1983 at 8.OO p.m. Where St. Matthews Hall, Bridge Street, Kensington. Subject: Photography and Cameras: renowned photographer Mr Alwyn Clements will explain the finer points of photographing native orchids or wildflowers for that matter. If you have had trouble getting those blooming plants in focus help is at hand - a pen and notepad may be useful items to bring along. LAST MEETING Last meeting Reg Shooter, our vice president, gave us an excellent illus- trated talk on how he grows dendrobiums. His talk was full of down to earth information. I cannot do full justice to him in a few words: a feature article will appear in the near future. Many thanks Reg, I will certainly update my own cultural methods. ALTERATIONS TO CONSTITUTION Both proposed alterations were passed unanimously (see February Journal for details). 22 LIFE MEMBERSHIP Our hard-working Secretary Roy Hargreaves has the honour of having the first Life Membership of this Society bestowed upon him by unanimous decision at the 1983 Annual General Meeting. -

Fire Responses of Bushland Plants After the January 1994 Wildfires in Northern Sydney
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Hochschulschriftenserver - Universität Frankfurt am Main Fire responses of bushland plants after the January 1994 wildfires in northern Sydney P.J.Kubiak P.O. Box 439, Ryde, NSW 1680 AUSTRALIA Abstract: In early January 1994 wildfires burned areas of bushland in northern Sydney (lat 33° 45’ S, long 151° 05’ E) in coastal south-eastern Australia. This paper reports observations of the fire responses for 828 species of bushland plants – 576 native species and 252 exotic species in the Lane Cove River and Narrabeen Lagoon catchment areas. Information recorded includes whether a species was killed by fire or resprouted post-fire, when seedlings were first observed following fire, and the times of first flowering and first fruiting (or spore production) after the fires. The estimated peaks of post-fire flowering or fruiting for a few species are given. It was not practicable to record data in all categories for all of the 828 species due to the logistical challenges involved in recording data across a large area of bushland, over a number of years. The data presented add to the growing body of knowledge on plant fire responses and will assist the management and conservation of bushland in the study areas, as well as the broader Sydney region. Cunninghamia (2009) 11(1): 131–165 Introduction Following a fire, the time taken by plants to flower after germination from seed, is known as the ‘primary juvenile Fire plays an important role in the shaping of Australia’s period’. -

December 2018 Bulletin
June, 2019 Bulletin Editor: Mike Hitchcock Next Meeting: Bulletin Th [email protected] 10 June 2019 Next meeting: 13th May, 2019 Placegetters at the May Meeting Paph spicerianum • Plant of the Night • Species Plant of the Night • Exhibited by Jan Robinson Rlc Village Chief North 'Green Genius' • Hybrid of Night • Exhibited by Peter Ng Paph Maudiae • Novice Plant Of The Night • Exhibited by P Martin & P Fink Howeara Lava Burst ‘puananii’ • Intermediate Plant Of The Night • Exhibited by Robert Crawley Whats Happening th Winter Show June 13, 14, and 15 (set up on June 12 ) st th Spring Show August 29, 30 and 31 (set up on Aug 28 ) St Ives Orchid Fair – 16th – 18th August St Ives Showground St Ives Southern Orchid Spectacular/Sharkies/Baskets October 11, 12, 13 (set up on Oct 10) April Monthly Meeting Minutes Without a Secretary we have no Monthly Meeting Minutes for the April meeting Monthly Speakers JUNE: Gifford Bunt on orchids in Vietnam from his recent trip JULY: Chris Dalrymple on What problem is that? AUG: Gary Hodder to talk on speciosums SEPT: OCT: Jeanne Dunn from Passion Orchids to talk on dockrillias (TBC) MONTHLY JUDGING RESULTS For : May 2019 PLANT OF NIGHT Winner Paph. spicerianum 'Jumbo' J. Robinson OPEN CLASS HYBRID Winner Blc. Village Chief North 'Green Genius' P. Ng OPEN CLASS SPECIES Winner Paph. spicerianum 'Jumbo' J. Robinson INTERMEDIATE JUDGES CHOICE Winner Leomesezia Lava Burst 'Panani' R. Cawley PRESIDENT'S CHOICE Winner Bulb. laxiflorum M. Hitchcock CLASS # 1 AUSTRALIAN NATIVES 1st Den. rigidum 'Dennis' C. Brandon 2nd Bulb. shepherdii I. -
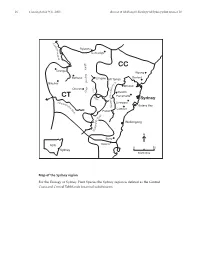
Part 10 ESP Intro
16 Cunninghamia 9(1): 2005 Benson & McDougall, Ecology of Sydney plant species 10 M a c q u Rylstone a r i e Coricudgy R i v e r e g n CC a Orange R Wyong g n i Gosford Bathurst d i Lithgow v Mt Tomah i Blayney D R. y r Windsor C t u a o b Oberon s e x r e s G k Penrith w a R Parramatta CT H i ve – Sydney r n a Abe e Liverpool rcro p m e b Botany Bay ie N R Camden iv Picton er er iv R y l l i Wollongong d n o l l o W N Berry NSW Nowra 050 Sydney kilometres Map of the Sydney region For the Ecology of Sydney Plant Species the Sydney region is defined as the Central Coast and Central Tablelands botanical subdivisions. Cunninghamia 9(1): 2005 Benson & McDougall, Ecology of Sydney plant species 10 17 Ecology of Sydney plant species Part 10 Monocotyledon families Lemnaceae to Zosteraceae Doug Benson and Lyn McDougall Royal Botanic Gardens and Domain Trust, Sydney, AUSTRALIA 2000. Email: [email protected] Abstract: Ecological data in tabular form are provided on 668 plant species of the families Lemnaceae to Zosteraceae, 505 native and 163 exotics, occurring in the Sydney region, defined by the Central Coast and Central Tablelands botanical subdivisions of New South Wales (approximately bounded by Lake Macquarie, Orange, Crookwell and Nowra). Relevant Local Government Areas are Auburn, Ashfield, Bankstown, Bathurst, Baulkham Hills, Blacktown, Blayney, Blue Mountains, Botany, Burwood, Cabonne, Camden, Campbelltown, Canada Bay, Canterbury, Cessnock, Crookwell, Evans, Fairfield, Greater Lithgow, Gosford, Hawkesbury, Holroyd, Hornsby, Hunters Hill, Hurstville, Kiama, Kogarah, Ku-ring-gai, Lake Macquarie, Lane Cove, Leichhardt, Liverpool, Manly, Marrickville, Mosman, Mulwaree, North Sydney, Oberon, Orange, Parramatta, Penrith, Pittwater, Randwick, Rockdale, Ryde, Rylstone, Shellharbour, Shoalhaven, Singleton, South Sydney, Strathfield, Sutherland, Sydney City, Warringah, Waverley, Willoughby, Wingecarribee, Wollondilly, Wollongong, Woollahra and Wyong. -

Indigenous Orchid Study Group
INDIGENOUS ORCHID STUDY GROUP Group Leaders: Don & Pauline Lawie P 0 Box 230 BABINDA Qld 4861 Phone: 07 40 679 577 Pauline is away this month attending the birthday of our only grandchild in Brisbane, where we have three married daughters, then going on with them to visit her siblings in N.S.W. That leaves me with an open palette for this edition, which means no pretty colour pictures (or italics) this time. We hope to return to colour next issue and to feature more of Kate Vlcek's impressive work. This is our 41st Newsletter and looking back through previous years I see that our style and content have changed over the years: new technology and Pauline's computer expertise has improved the presentation, but content always depends on feedback and contributions from members. As we send off each edition I wonder just how many will be opened and read with interest, or how many will be cast aside for later reading then forgotten, or simply filed, unread. We get very little comment, favourable or otherwise, on our efforts; we welcome both sorts. One reaction that pleases us is the effort that various state study group liasion officers go to to publicise the work of the various study groups, Most state SGAP/APS bulletins feature a page : "News from the study groups" which include short precis of recent study group newsletters. This gives the group's activities a wider audience and hopefully, new members. Our sincere thanks to those state officers who perform this service to us. Some follow-up on last newsletter: The front-page colour picture really grabbed attention: I think that there's some relationship there to the major article later in this newsletter. -

Native Orchid Society South Australia
NATIVE ORCHID SOCIETY SOUTH AUSTRALIA JOURNAL NATIVE ORCHID SOCIETY OF SOUTH AUSTRALIA Volume 7, No. 3 April 1983 Price 500 Registered by Australia Post Publication No. 38H 1344 PATRON: Mr T.R.N. Lothian PRESIDENT Mr G.J. Qieuuenhovon SECRETARY Mr E.R. Hargreaves 15 Robin Terrace 1 Halmon Avenue HOPE VALLEY S.A. 5090 EVERARDPARK S.A. 5075 Telephone 264 5825 Telephone 293 2471 297 3724 VICE PRESIDENT Mr R. Shooter COMMITTEE Mrs M. Fuller TREASURER Mr R " T ^ Robjohns Mr R. Bates Mr W. Harris EDITOR Mr G.O. Nieuwenhoven Mr P. Barnes NEXTMEETING Whey Tueodayv 26 April, 1983 at 8 ^ OO p.m. Where St. Matthews Hall, Bridge Streetq Kensington. Subject: Photography and Cameras renowned photographer Mr Alwyn Clements will explain the finer points of photographing native orchids or wildflowers for that matter. If you have had trouble getting those blooming plants in focus help is at hand -- a pen and notepad may be useful items to bring along. LAST MEETING Last meeting Reg Shooter, our vice preaidentg gave us an excellent illus- trated talk on how he grows dendrobiums. His talk was full of down to earth information ° I cannot do full justice to him in a few words a feature article will appear in the near future. Many thanks Reg, I will certainly update my own cultural methods. Both prnpoauo alterations were passed unanimously (see February Dc/ma, for details ` . 22° LIFE Our hard-working Secretary Roy Hargreaves has the honour of having the first Life Membership of this Society bestowed upon him by unanimous decision at the 1983 Annual General Meeting " I cannot think of a more deserving person to receive this award. -

台灣產羊耳蒜屬植物之分類研究A Taxonomic Study of Liparis LC Rich
⊕ 國立中山大學生物科學系 碩士論文 台灣產羊耳蒜屬植物之分類研究 A Taxonomic Study of Liparis L. C. Rich. (Orchidaceae) of Taiwan 研究生:楊智凱 指導教授:楊遠波 博士 中華民國九十五年六月 恩師楊遠波博士的教誨,嚴謹的治學態度及談吐中分享著多年研究經驗,在 研究上放心且信賴的任我盡情揮灑,生活上的關心與照顧,學生獲益匪淺。而遇 到瓶頸以及問題時老師總能一語道破,使論文進展更順利,在論文初稿撰寫期間 更不厭其煩多次費心的細心批改,讓其黑髮一夜髮白,心中萬分的感謝實在非三 言兩語可表達。任何事情保持著 `懷疑’的研究精神,更深深地烙印在學生心裡。 文稿初成,承蒙台灣大學森林環境暨資源學系蘇鴻傑教授在觀念與文獻上的 細心提點,成功大學生命科學系郭長生教授在百忙中撥空指教,論文之缺失不足 之處因兩位師長的指導而趨於完整,在此謹致由衷的謝意。 感謝英國皇家邱植物園標本館(K)Dr. Emma 協助拍攝大量之珍貴模式標 本;波蘭格但斯克大學植物分類與植物地理研究室 Dr. Margonska 提供寶貴文 獻;中央研究院植物標本館(HAST)彭鏡毅博士、林業試驗所標本館(TAIF) 邱文良博士、台灣大學植物標本館(TAI)郭城孟博士、台灣大學森林學系標本 館( NTUF)應紹順博士、台灣師範大學生物系植物標本館(TNU)王震哲博士、 自然科學博物館植物標本館(TNM)王秋美博士、屏東科技大學森林學系標本 館(PPI)楊勝任博士與特有生物研究保育中心植物標本館(TESRI)惠予借閱 標本並提供諸多協助以及林業試驗所森林保護組黃倩容學姐協助電子顯微鏡的 拍攝工作。 感謝林業試驗所鐘詩文博士諸多關照,慷慨提供日本東京大學植物標本館 (TI)的珍貴模式標本照片並熱心介紹日本筑波植物園蘭科專家 Dr. Yukawa , 其提供許多日本的寶貴文獻並告知研究成果,對我有很大助益,在此一併感謝。 研究得以順利進行都因為師長與好友們的幫忙。感謝系上昆蟲系統分類與演 化研究室顏聖紘博士,於研究初期幫忙影印大量的國外相關文獻並細心教導系統 分類學實務及支序地理學上的觀念。感謝研究室學長姐:怡姍、美珠、冠儀、東 哥、施兄、玉鳳、小旻、麗珍的指導與鼓勵,特別是小柯學長幫助我熟悉這個陌 生的環境;俊奎學長在生物繪圖上的教導;淑枝學姐於研究期間經費的安排與調 度;默詩學姐協助拍攝大量印尼茂物標本館(BO)標本;以誠學長協助拍攝中 國國家標本館(PE)模式標本;阿三哥在研究上及生活上的激勵以及關心以及 阿財學長在東部採集的細心照料。另外很感謝小胖幫忙鑑定昆蟲、怡玲、建炘等 諸位學弟妹幫忙協助實驗室計畫。 在植物分類及植物生態上的啟蒙特別感謝中興大學森林學系歐辰雄博士、呂 金誠博士、曾彥學博士,不僅在大學及研究所期間於學業與生活上的關心,歐老 師及呂老師更是在我遭遇困難時大力支持。生命科學系劉思謙博士,亦師亦友的 他在不僅在生活及研究上提供鼓勵與支持,創新的想法更給了我驚異激盪的空 間。也感謝森林學系森林植物分類暨生態研究室的學長姐,鴻志、朱哥、立彥、 志強、志銓、玄武、建志、政人、靖融、清安、俊凱、喜哥、芒果、國銘、傑鈞、 錦聰、家銘、秋瑩、詩婷、敏君的指導與鼓勵,讓我這段路走的更紮實,特別是 歐師母黃玲玉小姐,不管在生活上以及課業上都將我視如己出細心照顧,在此想 說一聲「謝謝你,歐師母。」 屏東科技大學森林學系謝光普同學從台中高農時期即互相砥礪與鼓勵,一起 堅持著我們走的路,沒有膽怯,這段同袍情誼將永遠留藏我心。 更感謝大學時期的摯友盈如、哀力、育揚、偉青、雅慧、碩容等的鼓勵和協 助;高中時期的摯友明益、老劉、原彰、小呈、麗芬、佳霖、以親等的陪伴。臺 -

Haagii: Listed for Botanist and Naturalist, Friedrich Adolph Haage (1796-1866)
haagii: listed for botanist and naturalist, Friedrich Adolph Haage (1796-1866). ex Colombia, cf. Cycnoches haagii Barb.Rodr.1881. habbemae: of Habbema, see habbemense. cf. Phreatia habbemae J.J.Sm.1910. habbemense: from Lake Habbema, central Papua, Indon. cf. Bulbophyllum habbemense P.Royen 1979. Noted as endemic. Habenaria: Willd.1805: ref. habena: a strap; thong + see -are: like, etc. Ref. the long thin lobes of the labellum. ex China, eg. Habenaria oligoschista. habenaria: strap; thong + like. cf. Orchis habenaria L. syn. Habenaria quinqueseta var. macroceratitis. Habenarieae: Habenaria + suffix denotes it’s a tribe. Habenariinae: Habenaria + suffix denotes it’s a subtribe. habenarina: strap + see -are: relative to + see -ina: like, etc. Or, more likely, the raceme was likened to that of a Habenaria. cf. Liparis habenarina. Noted as endemic to Aust. habenarioidea: strap-like + resembling, or see Habenaria + resembles. cf. Orchis habenarioidea. Was listed as such, but may have been an error for Orchis habenarioides (?). syn. Gymnadenia orchidis. habenarioides: Habenaria + resembling. cf. Pterichis habenarioides. x Habenari-orchis: Rolfe. On the syn. list, cf. Habenaria x Orchis. Habenella: Small 1903: ref. habena: thong; strap + suffix: small. Ref. the slender labellum lobes. syn. Habenaria Willd. habenifera: strap + bearing. cf. Lepanthes habenifera. Habenorkis: Thouars 1809: strap + orchid. See expl. for Habenaria. syn. Habenaria. habenula: small strap. cf. Pleurothallis habenula. habit: ref. habitus: condition; disposition (of life). Usually refs. to a peculiarity, feature, or distinction of the species under discussion. habitat: for a plant’s natural home: how and where it grows. Often seen as a heading, in a discussion, or protologue, giving a separate listing and description of the plant’s natural environment and ecology. -

A Preliminary List of Vascular Plant Species for Far South-Eastern New South Wales
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. RESEARCH PAPER NO. 4 19BB A Preliminary List of Vascular Plant Species for Far South-Eastern New South Wales by D. Binns 4 FORESTRV COMMISSION OF NEW SOUTH WALES A PRELIMINARY LIST OF VASCULAR PLANT SPECIES FOR FAR SOUTH-EASTERN NEW SOUTH WALES D. Binns WAUCHOPE RESEARCH CENTRE FORESTRY COMMISSION OF NEW SOUTH WALES FORESTRY COMMISSION OF NEW SOUTH WALES RESEARCH PAPER NO. 4 1988 Research Paper No. 4 1988 Mr D. Binns is a Research Forester based at the Wauchope Research Centre Published by: Forestry Commission of New South Wales Wood Technology and Forest Research Division 27 Oratava Avenue, West Pennant Hills, 2120 P.O. Box 100, Beecroft, 2119 Australia. Copyright: Forestry Commission of New South Wales; 1988 ODC 17(944) ISSN 0729-5340 ISBN 07305 19023 INTRODUCTION data at the phytosociological level, or to relate species distribution to environmental Relative to other parts of eastern New factors. The available data are unsuitable South Wales, the far south-east has been and insufficient for a comprehensive poorly explored botanically and little treatment of that type. floristic information has been available. Only two floristic lists have been cited for this area (Pickard, 1972; Bryant and ARRANGEMENT Benson, 1981).