University of Southampton Research Repository
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylum MOLLUSCA Chitons, Bivalves, Sea Snails, Sea Slugs, Octopus, Squid, Tusk Shell
Phylum MOLLUSCA Chitons, bivalves, sea snails, sea slugs, octopus, squid, tusk shell Bruce Marshall, Steve O’Shea with additional input for squid from Neil Bagley, Peter McMillan, Reyn Naylor, Darren Stevens, Di Tracey Phylum Aplacophora In New Zealand, these are worm-like molluscs found in sandy mud. There is no shell. The tiny MOLLUSCA solenogasters have bristle-like spicules over Chitons, bivalves, sea snails, sea almost the whole body, a groove on the underside of the body, and no gills. The more worm-like slugs, octopus, squid, tusk shells caudofoveates have a groove and fewer spicules but have gills. There are 10 species, 8 undescribed. The mollusca is the second most speciose animal Bivalvia phylum in the sea after Arthropoda. The phylum Clams, mussels, oysters, scallops, etc. The shell is name is taken from the Latin (molluscus, soft), in two halves (valves) connected by a ligament and referring to the soft bodies of these creatures, but hinge and anterior and posterior adductor muscles. most species have some kind of protective shell Gills are well-developed and there is no radula. and hence are called shellfish. Some, like sea There are 680 species, 231 undescribed. slugs, have no shell at all. Most molluscs also have a strap-like ribbon of minute teeth — the Scaphopoda radula — inside the mouth, but this characteristic Tusk shells. The body and head are reduced but Molluscan feature is lacking in clams (bivalves) and there is a foot that is used for burrowing in soft some deep-sea finned octopuses. A significant part sediments. The shell is open at both ends, with of the body is muscular, like the adductor muscles the narrow tip just above the sediment surface for and foot of clams and scallops, the head-foot of respiration. -

The Lower Bathyal and Abyssal Seafloor Fauna of Eastern Australia T
O’Hara et al. Marine Biodiversity Records (2020) 13:11 https://doi.org/10.1186/s41200-020-00194-1 RESEARCH Open Access The lower bathyal and abyssal seafloor fauna of eastern Australia T. D. O’Hara1* , A. Williams2, S. T. Ahyong3, P. Alderslade2, T. Alvestad4, D. Bray1, I. Burghardt3, N. Budaeva4, F. Criscione3, A. L. Crowther5, M. Ekins6, M. Eléaume7, C. A. Farrelly1, J. K. Finn1, M. N. Georgieva8, A. Graham9, M. Gomon1, K. Gowlett-Holmes2, L. M. Gunton3, A. Hallan3, A. M. Hosie10, P. Hutchings3,11, H. Kise12, F. Köhler3, J. A. Konsgrud4, E. Kupriyanova3,11,C.C.Lu1, M. Mackenzie1, C. Mah13, H. MacIntosh1, K. L. Merrin1, A. Miskelly3, M. L. Mitchell1, K. Moore14, A. Murray3,P.M.O’Loughlin1, H. Paxton3,11, J. J. Pogonoski9, D. Staples1, J. E. Watson1, R. S. Wilson1, J. Zhang3,15 and N. J. Bax2,16 Abstract Background: Our knowledge of the benthic fauna at lower bathyal to abyssal (LBA, > 2000 m) depths off Eastern Australia was very limited with only a few samples having been collected from these habitats over the last 150 years. In May–June 2017, the IN2017_V03 expedition of the RV Investigator sampled LBA benthic communities along the lower slope and abyss of Australia’s eastern margin from off mid-Tasmania (42°S) to the Coral Sea (23°S), with particular emphasis on describing and analysing patterns of biodiversity that occur within a newly declared network of offshore marine parks. Methods: The study design was to deploy a 4 m (metal) beam trawl and Brenke sled to collect samples on soft sediment substrata at the target seafloor depths of 2500 and 4000 m at every 1.5 degrees of latitude along the western boundary of the Tasman Sea from 42° to 23°S, traversing seven Australian Marine Parks. -

An Isotope Approach
Determining Cetacean – Cephalopod Trophic Interactions – An Isotope Approach By Christine Heather Jackson Bachelor of Arts, James Cook University Graduate Diploma (Health Promotion), Curtin University of Technology Master of Science, University of Tasmania Submitted in fulfilment of the requirements for the degree Doctor of Philosophy University of Tasmania (October 2016) ii DECLARATION This thesis contains no material which has been accepted for a degree or diploma by the University or any other institution, except by way of background information and duly acknowledged in the thesis, and to the best of my knowledge and belief no material previously published or written by another person except where due acknowledgement is made in the text of the thesis, nor does the thesis contain any material that infringes copyright Signed; _________________________________ Christine H. Jackson iii AUTHORITY OF ACCESS This thesis may be made available for loan and limited copying and communication in accordance with the Copyright Act 1968. Signed; _________________________________ Christine H. Jackson iv ABSTRACT Determining the biotic and abiotic influences on the distribution and abundance of marine mammals is essential for understanding the dynamics of the food chain. The predator-prey relationship can be deterministic in shaping both the community structure and function of marine ecosystems. This is especially pertinent to recovering toothed whale populations given their large size and high prey consumption rates. A greater knowledge of the trophic linkages between toothed whales and their prey will facilitate assessments of their combined impact on the ecosystem since marine food webs are a fusion of bottom-up and top-down energy and nutrient flow. -

Mastigotragus, a New Generic Name for Mastigoteuthis Pyrodes Young, 1972 (Cephalopoda: Mastigoteuthidae)
European Journal of Taxonomy 105: 1–6 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.105 www.europeanjournaloftaxonomy.eu 2014 · Young R.E., Vecchione M. & Braid H.E. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:FCD51F6F-A5D6-4466-ADB6-0ADC9F560F66 Mastigotragus, a new generic name for Mastigoteuthis pyrodes Young, 1972 (Cephalopoda: Mastigoteuthidae) Richard E. YOUNG,1 Michael VECCHIONE 2 & Heather E. BRAID 3 1 University of Hawaii, Honolulu, Hawaii, USA. Email: [email protected] 2 NMFS National Systematics Laboratory, Smithsonian Institution, Washington, DC, USA. Email: [email protected] 3 Institute for Applied Ecology New Zealand, Auckland University of Technology, Auckland, New Zealand. Email: [email protected] 1 urn:lsid:zoobank.org:author:C5177553-E350-4C6A-9D2E-70581F2ED152 2 urn:lsid:zoobank.org:author:E79EA1DA-A7B4-482D-A5E3-AB81E74E4743 3 urn:lsid:zoobank.org:author:A28B5D88-B00C-46A1-9F48-367918CAF2F2 Abstract. A recent paper on the phylogenetic relationships of species within the cephalopod family Mastigoteuthidae meant great progress in stabilizing the classifi cation of the family. The authors, however, left the generic placement of Mastigoteuthis pyrodes unresolved. This problem is corrected here by placing this species in a new monotypic genus, Mastigotragus, based on unique structures of the photophores and the funnel/mantle locking apparatus. Keywords. Mastigoteuthidae, morphology, nomenclature, Mastigotragus gen. nov., deep-sea squid Young R.E., Vecchione M. & Braid H.E. 2014. Mastigotragus, a new generic name for Mastigoteuthis pyrodes Young, 1972 (Cephalopoda: Mastigoteuthidae). European Journal of Taxonomy 105: 1–6. http://dx.doi. -

Applying New Tools to Cephalopod Trophic Dynamics and Ecology: Perspectives from the Southern Ocean Cephalopod Workshop, February 2–3, 2006
Rev Fish Biol Fisheries (2007) 17:79–99 DOI 10.1007/s11160-007-9055-9 RESEARCH PAPER Applying new tools to cephalopod trophic dynamics and ecology: perspectives from the Southern Ocean Cephalopod Workshop, February 2–3, 2006 G. D. Jackson Æ P. Bustamante Æ Y. Cherel Æ E. A. Fulton Æ E. P. M. Grist Æ C. H. Jackson Æ P. D. Nichols Æ H. Pethybridge Æ K. Phillips Æ R. D. Ward Æ J. C. Xavier Received: 30 October 2006 / Accepted: 14 February 2007 / Published online: 28 March 2007 Ó Springer Science+Business Media B.V. 2007 Abstract A two day workshop on Southern the ecosystem by incorporating squid data in Ocean cephalopods was held in Hobart, Tasmania, ecosystem models. Genetic barcoding is now of Australia prior to the triennial 2006 Cephalopod great value to fish taxonomy as well as other groups International Advisory Council (CIAC) sympo- and it is expected that a cephalopod barcoding sium. The workshop provided a second interna- initiative will be an important tool for cephalopod tional forum to present the current state of taxonomy. There is a current initiative to produce research and new directions since the last Southern a new cephalopod beak identification guide to Ocean cephalopod meeting held in 1993. A major assist predator biologists in identifying cephalopod focus of the workshop was trophic ecology and the prey items. There were also general discussions on use of a variety of tools that can be applied in specific taxonomic issues, Southern Ocean Ceph- Southern Ocean trophic studies for both cephalo- alopod paralarvae and parasites, and suggestions pod and predator researchers. -

Mastigotragus, a New Generic Name for Mastigoteuthis Pyrodes Young
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: European Journal of Taxonomy Jahr/Year: 2014 Band/Volume: 0105 Autor(en)/Author(s): Young Richard E., Vecchione Michael, Braid Heather E. Artikel/Article: Mastigotragus, a new generic name for Mastigoteuthis pyrodes Young, 1972 (Cephalopoda: Mastigoteuthidae) 1-6 European Journal of Taxonomy 105: 1–6 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.105 www.europeanjournaloftaxonomy.eu 2014 · Young R.E., Vecchione M. & Braid H.E. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:FCD51F6F-A5D6-4466-ADB6-0ADC9F560F66 Mastigotragus, a new generic name for Mastigoteuthis pyrodes Young, 1972 (Cephalopoda: Mastigoteuthidae) Richard E. YOUNG,1 Michael VECCHIONE 2 & Heather E. BRAID 3 1 University of Hawaii, Honolulu, Hawaii, USA. Email: [email protected] 2 NMFS National Systematics Laboratory, Smithsonian Institution, Washington, DC, USA. Email: [email protected] 3 Institute for Applied Ecology New Zealand, Auckland University of Technology, Auckland, New Zealand. Email: [email protected] 1 urn:lsid:zoobank.org:author:C5177553-E350-4C6A-9D2E-70581F2ED152 2 urn:lsid:zoobank.org:author:E79EA1DA-A7B4-482D-A5E3-AB81E74E4743 3 urn:lsid:zoobank.org:author:A28B5D88-B00C-46A1-9F48-367918CAF2F2 Abstract. A recent paper on the phylogenetic relationships of species within the cephalopod family Mastigoteuthidae meant great progress in stabilizing the classifi cation of the family. The authors, however, left the generic placement of Mastigoteuthis pyrodes unresolved. This problem is corrected here by placing this species in a new monotypic genus, Mastigotragus, based on unique structures of the photophores and the funnel/mantle locking apparatus. -

ASFIS ISSCAAP Fish List February 2007 Sorted on Scientific Name
ASFIS ISSCAAP Fish List Sorted on Scientific Name February 2007 Scientific name English Name French name Spanish Name Code Abalistes stellaris (Bloch & Schneider 1801) Starry triggerfish AJS Abbottina rivularis (Basilewsky 1855) Chinese false gudgeon ABB Ablabys binotatus (Peters 1855) Redskinfish ABW Ablennes hians (Valenciennes 1846) Flat needlefish Orphie plate Agujón sable BAF Aborichthys elongatus Hora 1921 ABE Abralia andamanika Goodrich 1898 BLK Abralia veranyi (Rüppell 1844) Verany's enope squid Encornet de Verany Enoploluria de Verany BLJ Abraliopsis pfefferi (Verany 1837) Pfeffer's enope squid Encornet de Pfeffer Enoploluria de Pfeffer BJF Abramis brama (Linnaeus 1758) Freshwater bream Brème d'eau douce Brema común FBM Abramis spp Freshwater breams nei Brèmes d'eau douce nca Bremas nep FBR Abramites eques (Steindachner 1878) ABQ Abudefduf luridus (Cuvier 1830) Canary damsel AUU Abudefduf saxatilis (Linnaeus 1758) Sergeant-major ABU Abyssobrotula galatheae Nielsen 1977 OAG Abyssocottus elochini Taliev 1955 AEZ Abythites lepidogenys (Smith & Radcliffe 1913) AHD Acanella spp Branched bamboo coral KQL Acanthacaris caeca (A. Milne Edwards 1881) Atlantic deep-sea lobster Langoustine arganelle Cigala de fondo NTK Acanthacaris tenuimana Bate 1888 Prickly deep-sea lobster Langoustine spinuleuse Cigala raspa NHI Acanthalburnus microlepis (De Filippi 1861) Blackbrow bleak AHL Acanthaphritis barbata (Okamura & Kishida 1963) NHT Acantharchus pomotis (Baird 1855) Mud sunfish AKP Acanthaxius caespitosa (Squires 1979) Deepwater mud lobster Langouste -

A New Microendemic Species of the Deep-Water Catshark Genus Bythaelurus
RESEARCH ARTICLE A new microendemic species of the deep- water catshark genus Bythaelurus (Carcharhiniformes, Pentanchidae) from the northwestern Indian Ocean, with investigations of its feeding ecology, generic review and identification key 1,2 3 2 Simon WeigmannID *, Carina Julia KaschnerID , Ralf Thiel a1111111111 1 Elasmo-Lab, Elasmobranch Research Laboratory, Hamburg, Germany, 2 Center of Natural History, a1111111111 University of Hamburg, Hamburg, Germany, 3 Department 522, Fisheries, Federal Office for Agriculture and a1111111111 Food, Hamburg, Germany a1111111111 a1111111111 * [email protected] Abstract OPEN ACCESS A new deep-water catshark, Bythaelurus stewarti, is described based on 121 examined Citation: Weigmann S, Kaschner CJ, Thiel R specimens caught on the Error Seamount (Mount Error Guyot) in the northwestern Indian (2018) A new microendemic species of the deep- Ocean. The new species differs from all congeners in the restricted distribution, a higher spi- water catshark genus Bythaelurus ral valve turn count and in the morphology of the dermal denticles. It is distinguished from its (Carcharhiniformes, Pentanchidae) from the northwestern Indian Ocean, with investigations of morphologically and geographically closest congener, B. hispidus (Alcock), by the larger its feeding ecology, generic review and size (maximum size 44 vs. 39 cm TL, maturity size of males 35±39 vs. 21±28 cm TL), darker identification key. PLoS ONE 13(12): e0207887. fresh coloration and dark grayish-brown mottling of the ventral head (vs. ventral head typi- https://doi.org/10.1371/journal.pone.0207887 cally uniformly yellowish or whitish). Furthermore, it has a strongly different morphology of Editor: Ulrike Gertrud Munderloh, University of dermal denticles, in particular smaller and less elongate branchial, trunk and lateral caudal Minnesota, UNITED STATES denticles that are set much less densely and have a surface that is very strongly and fully Received: August 24, 2018 structured by reticulations (vs. -

And Their Functional, Ecological, and Evolutionary Implications
DePaul University Via Sapientiae College of Science and Health Theses and Dissertations College of Science and Health Spring 6-14-2019 Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications Phillip C. Sternes DePaul University, [email protected] Follow this and additional works at: https://via.library.depaul.edu/csh_etd Part of the Biology Commons Recommended Citation Sternes, Phillip C., "Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications" (2019). College of Science and Health Theses and Dissertations. 327. https://via.library.depaul.edu/csh_etd/327 This Thesis is brought to you for free and open access by the College of Science and Health at Via Sapientiae. It has been accepted for inclusion in College of Science and Health Theses and Dissertations by an authorized administrator of Via Sapientiae. For more information, please contact [email protected]. Body Forms in Sharks (Chondrichthyes: Elasmobranchii), and Their Functional, Ecological, and Evolutionary Implications A Thesis Presented in Partial Fulfilment of the Requirements for the Degree of Master of Science June 2019 By Phillip C. Sternes Department of Biological Sciences College of Science and Health DePaul University Chicago, Illinois Table of Contents Table of Contents.............................................................................................................................ii List of Tables..................................................................................................................................iv -

Sperm Whale Diet in New Zealand
Thesis submitted to Auckland University of Technology in partial fulfilment of the degree of Master of Applied Science Sperm whale diet in New Zealand Felipe Gómez-Villota 2007 Table of contents Attestation of authorship .................................................................................... i List of abbreviations.......................................................................................... ii Acknowledgements............................................................................................ i Abstract ........................................................................................................... iii Introduction.........................................................................................................2 1.1 Objectives and thesis structure...................................................................3 1.2 Thesis outline..............................................................................................4 Literature review .................................................................................................8 2.1 Sperm whale biology ..................................................................................8 2.1.1 Anatomy of the sperm whale.............................................................8 2.1.2 Distribution and migration ...............................................................12 2.1.3 Social structure ...............................................................................15 2.1.4 Diving behaviour .............................................................................17 -

Report of the Workshop on Deep-Sea Species Identification, Rome, 2–4 December 2009
FAO Fisheries and Aquaculture Report No. 947 FIRF/R947 (En) ISSN 2070-6987 Report of the WORKSHOP ON DEEP-SEA SPECIES IDENTIFICATION Rome, Italy, 2–4 December 2009 Cover photo: An aggregation of the hexactinellid sponge Poliopogon amadou at the Great Meteor seamount, Northeast Atlantic. Courtesy of the Task Group for Maritime Affairs, Estrutura de Missão para os Assuntos do Mar – Portugal. Copies of FAO publications can be requested from: Sales and Marketing Group Office of Knowledge Exchange, Research and Extension Food and Agriculture Organization of the United Nations E-mail: [email protected] Fax: +39 06 57053360 Web site: www.fao.org/icatalog/inter-e.htm FAO Fisheries and Aquaculture Report No. 947 FIRF/R947 (En) Report of the WORKSHOP ON DEEP-SEA SPECIES IDENTIFICATION Rome, Italy, 2–4 December 2009 FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2011 The designations employed and the presentation of material in this Information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views of FAO. -
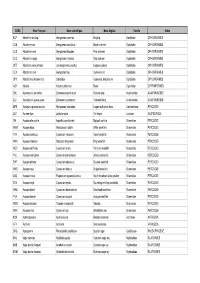
Nom Français
CODE Nom Français Nom scientifique Nom Anglais Famille Ordre KCP Abadèche du Cap Genypterus capensis Kingklip Ophidiidae OPHIDIIFORMES CUB Abadèche noir Genypterus maculatus Black cusk-eel Ophidiidae OPHIDIIFORMES CUS Abadèche rosé Genypterus blacodes Pink cusk-eel Ophidiidae OPHIDIIFORMES CUC Abadèche rouge Genypterus chilensis Red cusk-eel Ophidiidae OPHIDIIFORMES OFZ Abadèche sans jambes Lamprogrammus exutus Legless cuskeel Ophidiidae OPHIDIIFORMES CEX Abadèches nca Genypterus spp Cusk-eels nei Ophidiidae OPHIDIIFORMES OPH Abadèches, brotules nca Ophidiidae Cusk-eels, brotulas nei Ophidiidae OPHIDIIFORMES ALR Ablette Alburnus alburnus Bleak Cyprinidae CYPRINIFORMES ZML Acanthure à pierreries Zebrasoma gemmatum Spotted tang Acanthuridae ACANTHUROIDEI ZLV Acanthure à queue jaune Zebrasoma xanthurum Yellowtail tang Acanthuridae ACANTHUROIDEI MPS Achigan à grande bouche Micropterus salmoides Largemouth black bass Centrarchidae PERCOIDEI LQT Acmée râpe Lottia limatula File limpet Lottiidae GASTROPODA ISA Acoupa aile-courte Isopisthus parvipinnis Bigtooth corvina Sciaenidae PERCOIDEI WEW Acoupa blanc Atractoscion nobilis White weakfish Sciaenidae PERCOIDEI YNV Acoupa cambucu Cynoscion virescens Green weakfish Sciaenidae PERCOIDEI WKK Acoupa chasseur Macrodon ancylodon King weakfish Sciaenidae PERCOIDEI WEP Acoupa du Pérou Cynoscion analis Peruvian weakfish Sciaenidae PERCOIDEI YNJ Acoupa mongolare Cynoscion jamaicensis Jamaica weakfish Sciaenidae PERCOIDEI SWF Acoupa pintade Cynoscion nebulosus Spotted weakfish Sciaenidae PERCOIDEI WKS Acoupa