Iodine Status of Pregnant and Lactating Women in Arusha
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

National Environment Management Council (Nemc)
NATIONAL ENVIRONMENT MANAGEMENT COUNCIL (NEMC) NOTICE TO COLLECT APPROVED AND SIGNED ENVIRONMENTAL CERTIFICATES Section 81 of the Environment Management Act, 2004 stipulates that any person, being a proponent or a developer of a project or undertaking of a type specified in Third Schedule, to which Environmental Impact Assessment (EIA) is required to be made by the law governing such project or undertaking or in the absence of such law, by regulation made by the Minister, shall undertake or cause to be undertaken, at his own cost an environmental impact assessment study. The Environmental Management Act, (2004) requires also that upon completion of the review of the report, the National Environment Management Council (NEMC) shall submit recommendations to the Minister for approval and issuance of certificate. The approved and signed certificates are returned to NEMC to formalize their registration into the database before handing over to the Developers. Therefore, the National Environment Management Council (NEMC) is inviting proponents/developers to collect their approved and signed certificates in the categories of Environmental Impact Assessment, Environmental Audit, Variation and Transfer of Certificates, as well as Provisional Environmental Clearance. These Certificates can be picked at NEMC’s Head office at Plot No. 28, 29 &30-35 Regent Street, Mikocheni Announced by: Director General, National Environment Management Council (NEMC), Plot No. 28, 29 &30-35 Regent Street, P.O. Box 63154, Dar es Salaam. Telephone: +255 22 2774889, Direct line: +255 22 2774852 Mobile: 0713 608930/ 0692108566 Fax: +255 22 2774901, Email: [email protected] No Project Title and Location Developer 1. Construction of 8 storey Plus Mezzanine Al Rais Development Commercial/Residential Building at plot no 8 block Company Ltd, 67, Ukombozi Mtaa in Jangwani Ward, Ilala P.O. -

Tanzania Inventory Report-1 for Arusha Tana
UNITED REPUBLIC OF TANZANIA VICE PRESIDENT’S OFFICE INVENTORY OF THE WASTE OPEN BURNING IN ARUSHA, TANGA AND DAR ES SALAAM CITIES DECEMBER 2018 CONTENTS 1.0 INTRODUCTION ....................................................................................... 1 1.1 Background ........................................................................................................................... 1 1.2 Objectives of the site visit .................................................................................................. 1 2.0 APPROACH AND METHODS ...................................................................... 1 2.1 Appointment of the Team of Experts ............................................................................... 1 2.2 Site visit arrangement ......................................................................................................... 1 3.0 FINDINGS AND OBSERVATIONS ............................................................. 2 3.1 Existing situation.................................................................................................................. 2 3.1.1 Arusha City Council ................................................................................................. 2 3.1.2 Tanga City Counci lCouncil……………………………………..................................................7 3.1.3 Dar es Salaam City Council ................................................................................. 11 4.0 CONCLUSION……………………………………………………………………………………………………………….20 ' ANNEXES ........................................................................................................ -

Table of Contents
TABLE OF CONTENTS Arusha City Council | Five Years Strategic Plan – 2016/2017 – 2020/2021 Page 1 Abbreviations TSD Cord - Teachers Service Department Coordinator WE - Water Engineer CALDO - City Agriculture and Livestock Development Officer LO - Land Officer CNRO - City Natural Resources Officer ROSA - Resource Oriented sanitation MVC - Most Vulnerable Children C/MENG - Municipal/City Engineer BE - Building Engineer RAS - Regional Administrative Secretariat IT - Information Technology MKURABITA – Mkakati wa Kuratibisha Rasilimali PLHIV - People Living with HIV AUWSA - Arusha Urban water Supply and Sewerage Authority MSO/CSO - Municipal/City Supplies officer MS/CS - Municipal/City Solicitor IA - Internal Auditor MECOM/CICOM - Municipal/City Economic Officer MT/CT - Municipal/City Treasurer MTO/CTO - Municipal/City Trade Officer CMOOH - City Medical Officer of Health CCDO - City Community Development Officer CEO(S) - City Education Officer (Secondary) NMS - National Minimum Standards CHMT - Council Health Management Team MEK - Mratibu Elimu Kata TSD - Teachers Service Department OUT - Open University of Tanzania UCC - University Computing Centre IAA - Institute of Accountancy Arusha VETA - Vocational Education Training Agency SLO - Statistics and Logistic Officer HRO - Human resource Officer CEO (P) - City Education Officer (Primary) Std - Standard DFT - District facilitation Team WFP - World Food Programme WFT - Ward Facilitation Team TSCP - Tanzania Strategic Cities Project TAHA - Tanzania Horticulture Association Arusha City Council | Five Years Strategic Plan – 2016/2017 – 2020/2021 Page 2 PMO-RALG – Prime Minister’s Office Regional Administration and Local Government MoFE - Ministry of Finance CBOs - Community Based Organizations ICT - Information Communication Technology CCP - Chuo Cha Polisi HoDs - Heads of Department AG - Attorney General WEO - ward Executive officer WDC - Ward Development Committee PMU - Procurement management Unit PPRA - Public Procurement regulatory Authority PSPTB - Procurement and Supply Pro. -

Factors Influencing Academic Performance of Primary School's
Vol-6 Issue-4 2020 IJARIIE-ISSN(O)-2395-4396 Factors Influencing Academic Performance of Primary School’s Orphans in Tanzania Anthony Nyangarika and Zacharia Joseph Ngasa Department of Adult and Continuing Education Studies, Institute of Adult Education, P.O. Box 20679, Dar es Salaam, Tanzania ABSTRACT This study investigates the factor influencing orphan’s academic performance in primary schools. In Tanzania the number of orphaned students resulted from different causes such as the death of parents from different accidents, diseases and those with divorced parents is drastic increases. Orphaned students are among the most vulnerable members of society, often lacking basic needs and services related to better education. This study investigated the factors influencing the academic performance of orphans in Arusha City. The study used descriptive research design and it was conducted in Arusha City to primary school orphans from different wards. Data were collected from 100 respondents including DEO, WEC, head teachers, class teachers, other teachers, orphans, guardians, and community through interviews. Findings show that orphanage students were performing poorly and this was contributed by many factors including social-economic factors, home learning environment, sexual violence, inferiority complex, school’s absenteeism, lack of a system of helping orphaned students in schools. It was recommended that Schools should find a means to identify orphaned students and their needs and provide necessary support including psychological and also encourage them to learn and provide necessary academic support when appropriate. The orphaned students should participate fully in the learning process, and the MoEVT and other Educational stakeholders should plan and establish regular seminars to teachers on how to help students. -

Economics of Urban Households' Cooking Fuel
ECONOMICS OF URBAN HOUSEHOLDS’ COOKING FUEL CONSUMPTION IN ARUSHA CITY, TANZANIA SEMMY MOKITI THADEO A DISSERTATION SUBMITTED IN PARTAIL FULFILMENT OF THE REQUIREMENT FOR THE DEGREE OF MASTER OF SCIENCE IN ENVIRONMENTAL AND NATURAL RESOURCE ECONOMICS OF SOKOINE UNIVERSITY OF AGRICULTURE. MOROGORO, TANZANIA. 2014 ii ABSTRACT The study was conducted in Arusha City, Tanzania. The aim of the study was to analyse the socio-economic factors that influence urban households’ choice of primary cooking fuel and its share to the total household expenditure. The specific focus of the study were (i) to investigate households’ cooking fuel(s) consumption pattern (ii) to analyse factors affecting households’ choice of cooking fuel (iii) to examine households’ cooking fuel consumption intensity (iv) to develop a Liquidified Petroleum Gas (LPG) consumption descriptive model. A cross sectional research design was adapted for this study. Primary data was collected using semi-structured questionnaires administered to 200 households. Furthermore, Interview and checklist were used to collect information from 2 key informants and 25 cooking fuel dealers. The data obtained was analysed using Descriptive Analysis, Binary Logistics Regression, and Log-Linear Regression. The findings suggested that there are four cooking fuels used and available in the study area (firewood, charcoal, Kerosene and LPG). The principal cooking fuels as stated by the respondents were charcoal and LPG at 35.5% and 57.5% respectively. Most households prefer to use LPG to charcoal at 97.7% and 11.6% respectively. The study further revealed that the choice of the principal cooking fuel is influenced by socio- economic and demographic factors such as education level (p <0.01), marital status (p <0.01), occupation of respondent (p <0.05), household size (p ˂0.05), Residence ownership (p <0.05), and age of respondent (p <0.05). -

GN. LAND RENTS REVIEW 2012.Pdf
LAND RENT RATES (Made Under Regulation 2(3)) ARUSHA REGION ARUSHA CITY COUNCIL S/N ZONE AREA USER OLD RATES PER NEW RATES PER M2 M2 1 CBD Commercial Atrea "E",Commercial Residential 100.00 300.00 Area Blocks A - S, Block 21 along Commercial/Residential 150.00 600.00 Conal Midletron Road Commercial/Service Trade/restraurant 300.00 1,500.00 2 Next to CBD Residential Area I and II Corridor Area, Residential 40.00 120.00 Sekei,East of Golf Course, Kijenge South, Hotel 77, Olorien (Block KK), Blocks F, HH and W Themi and Uzungunig Commercial/Residential 60.00 240.00 Commercial/Service Trade/restraurant 120.00 600.00 3 Outside CBDThe Stretch from Nduruma river to Residential 40.00 120.00 Sakina (200 meters zone) Commercial/Residential 60.00 240.00 Commercial/Service Trade/restraurant 120.00 600.00 4 Outside CBDThe whole area of Kaloleni, Levolosi Residential 40.00 120.00 and Block 21 Commercial/Residential 60.00 240.00 Commercial/Service Trade/restraurant 120.00 600.00 5 Outside CBD The whole area of Blocks A - C Njiro Residential 40.00 120.00 Commercial/Residential 60.00 240.00 Commercial/Service Trade/restraurant 120.00 600.00 1 6 Outside CBD Kijenge, Mashono, Kimandolu, Residential 25.00 75.00 Kwangulelo, Olorien (Block KK) Commercial/Residential 37.50 150.00 Commercial/Service Trade/restraurant 75.00 375.00 7 Outside CBD The whole area of Unga limited, Esso, Residential 25.00 75.00 Commercial/Residential 37.50 150.00 Commercial/Service Trade/restraurant 75.00 375.00 8 Outside CBD The whole area of Ngarenaro, Block Residential 15.00 45.00 -

Community Energy Initiatives (2007)
JIP Final Progress Report Community Energy Initiatives, Tanzania SOKONI ONE-SOMBETINI Community Energy Initiatives A Final JIP Report submitted by the JIP Team Section One: Background Information I. PROJECT DESCRIPTION A. Project title, names of project leaders and team members Project Title: Community Energy Initiatives Project Leaders: Dr. Stuart Graham, University of California, Berkeley SYLFF fellow Mr. Stephen Mutinda Mutiso, University of Nairobi SYLFF fellow Other team members: Ms. Anna Oursler, UC Berkeley and WODSTA Mr. Amanuleus Kibona, WODSTA Ms. Adelasia Divona, Universidad de Duesto SYLFF fellow Dr. Garrick Blalock, Cornell University Dr. Claudia Radel, Princeton University SYLFF fellow B. Activities, including the role of each team member During 2007-2008, the JIPTeam implemented a “joint social action project” in accordance with the following outline: “Energy resources are increasingly scarce in Tanzania. This project addresses human problems that stem from that scarcity, which affect rural women and their families and intends to provide energy options to people. The project team has organized a ‘Community Energy Resource Center’ in Sombetini, (Tanzania), consisting of a central building where women, girls and families gather in a communal setting to learn about energy- sustaining technologies. The project team is using various methodologies, including several different approaches, consisting of disseminating information, public demonstrations, group discussions, open office hours, JIP Team and Women Development For Science -

ARUSHA JIJI BWENI .Pdf
MKOA WA ARUSHA HALMASHAURI YA JIJI LA ARUSHA WANAFUNZI WALIOCHAGULIWA KUJIUNGA NA KIDATO CHA KWANZA 2021 A: SHULE ZA BWENI SHULE ZENYE UFAULU MZURI ZAIDI I: WAVULANA NAMBA YA HALMASHAUR SHULE HALMASHAURI S/N JINA LA MTAHINIWA SHULE ATOKAYO MTAHINIWA I ATOKAYO AENDAYO AENDAYO 1 20141468033 MELVIN MELANCE KINABO SHALOM ARUSHA CC KIBAHA KIBAHA TC 2 20141526317 PASTORY RICHARD MASSAWE ST.JUDE ARUSHA CC KIBAHA KIBAHA TC 3 20141468011 ALLAN RICHARD MATOLO SHALOM ARUSHA CC ILBORU ARUSHA DC SHULE ZA UFUNDI NAMBA YA HALMASHAUR SHULE HALMASHAURI S/N JINA LA MTAHINIWA SHULE ATOKAYO MTAHINIWA I ATOKAYO AENDAYO AENDAYO 1 20141629595 SHALOM MALAKI MAFIE GHATI MEMORIAL ARUSHA CC TANGA TECH TANGA CC 2 20141522409 JAMES RUMANYIKA KAJUMULO LUCKY VICENT ARUSHA CC TANGA TECH TANGA CC 3 20141508440 ATHUMAN JUMA ATHUMAN UPENDO FRIENDS ARUSHA CC TANGA TECH TANGA CC 4 20141465294 EBENEZA BERNARD SALIJA HADY ARUSHA CC IFUNDA TECH IRINGA DC 5 20170438836 REAGAN OSWALD MLAY IMANI SCHOOL ARUSHA CC IFUNDA TECH MOSHI MC 6 20141492353 ELISHA ALLAN HIZA ST. MONICA ARUSHA CC MOSHI TECH IRINGA DC 7 20141500555 IBRAHIM ALFRED KAPINGA NARIVA HILL ARUSHA CC TANGA TECH TANGA CC B: SHULE ZENYE UFAULU MZURI ZAIDI II:WASICHANA NAMBA YA HALMASHAUR SHULE HALMASHAURI S/N JINA LA MTAHINIWA SHULE ATOKAYO MTAHINIWA I ATOKAYO AENDAYO AENDAYO 1 20141526380 LILIAN JOSEPH MASHINA ST.JUDE ARUSHA CC KILAKALA MOROGORO MC 2 20141629665 SAMIRA HAUFI MUSTAFA GHATI MEMORIAL ARUSHA CC KILAKALA MOROGORO MC 3 20140246748 CONDOLEEZZA ELIREHEMA MOLLEL NARIVA HILL ARUSHA CC MSALATO DODOMA CC SHULE ZA UFUNDI NAMBA YA HALMASHAUR SHULE HALMASHAURI S/N JINA LA MTAHINIWA SHULE ATOKAYO MTAHINIWA I ATOKAYO AENDAYO AENDAYO 1 20141500572 JOANA ELIZAID MBAGA NARIVA HILL ARUSHA CC TANGA TECH TANGA CC C: WANAFUNZI WENYE MAHITAJI MAALUM WAVULANA NAMBA YA HALMASHAUR SEKONDARI HALMASHAURI Na. -

Andreasen Et Al 2017 Danish Journal of Geography Accepted Version Apr2017
Urban transformations, migration and residential mobility patterns in African secondary cities Andreasen, Manja Hoppe; Agergaard, Jytte; Robert, Kiunsi; Namangaya, Ally H Published in: Danish Journal of Geography DOI: 10.1080/00167223.2017.1326159 Publication date: 2017 Document version Peer reviewed version Citation for published version (APA): Andreasen, M. H., Agergaard, J., Robert, K., & Namangaya, A. H. (2017). Urban transformations, migration and residential mobility patterns in African secondary cities. Danish Journal of Geography, 117(2), 93-104. https://doi.org/10.1080/00167223.2017.1326159 Download date: 29. sep.. 2021 Andreasen M. H., J. Agergaard, R. B. Kiunsi and A. H. Namangaya (2017): Urban transformations, migration and residential mobility in Arusha, a secondary city of Tanzania. Geografisk Tidsskrift-Danish Journal of Geography 117(2): 93-104. https://doi.org/10.1080/00167223.2017.1326159. This manuscript is the version accepted for publication in April 2017. Urban transformations, migration and residential mobility patterns in Arusha, a secondary city of Tanzania Abstract Urban growth is a significant trend in Africa. Scholarly attention and urban planning efforts have focused disproportionately on the challenges of big cities, while small and medium-sized urban settlements are growing most rapidly and house the majority of urban residents. Small towns have received some attention, but very few studies have focused on secondary cities. This paper offers a study of urban transformations, migration and residential mobility patterns in Arusha, a rapidly growing secondary city of Tanzania. Arusha functions as a major attraction for migrants and in-migration is a central dynamic shaping transformation processes in central areas, which are characterized by high population turn-overs, vibrant rental markets and widespread landlordism. -

Contributing Factors to Early Pregnancy Among
ONTRIBUTING FACTORS TO EARLY PREGNANCY AMONG GIRLS IN PUBLIC SECONDARY SCHOOLS IN ARUSHA CITY JACQUELINE MARTINE KAPILEH A RESEARCH SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE AWARD OF MASTERS DEGREE OF ARTS IN MONITORING AND EVALUATION OF THE OPEN UNIVERSITY OF TANZANIA 2019 ii CERTIFICATION The undersigned certifies and hereby recommends for acceptance, by the Open University of Tanzania a dissertation titled; “Contributing factors to early pregnancy among girls in public secondary schools in Arusha city”, in partial fulfilment of the requirements for the degree of Master of Arts in Monitoring and Evaluation of the Open University of Tanzania. ……………….…………………. Dr. Harrieth Mtae (Supervisor) …………………………… Date iii COPYRIGHT No part of this research may be reproduced, stored in any retrieval system or transmitted in any form by any means, electronic, mechanical, photocopying, recording or otherwise without a prior written permission of the author or The Open University of Tanzania in that behalf. iv DECLARATION I, Jacqueline Martine Kapileh, do hereby declared that this research report is my own original work, and that it has not been submitted and was not submitted for the similar or other degree award in any University. ……………………................ Signature ……………………………. Date v DEDICATION I dedicate this work to my family. vi ACKNOWLEDGEMENT In accomplishing this Dissertation, first and foremost, I thank the Almighty God for his Love and strengths He has given me as from the beginning to the completion of this work. I wish to express my sincere appreciation to all those who contributed in various ways in the enhancement of sources of this work. In a very special way, I would like to extend special gratitude to my supervisor Dr. -

List of Licensed Petrol Station in Tanzania As of Aug
LIST OF LICENSED PETROL STATION IN TANZANIA AS OF AUG. 2014 S/No. Name of the Petrol Station Region District Location Plot No.122, Block 1 Sombetini Service Station Arusha Arusha No. "EE", Ngarenaro Plot No. 452/453, Panone and Company Ltd- Block"Y", 2 Arusha Arusha Engarenarok Filling Station Engarenarok in Arusha Municipality Puma Energy Tanzania - Market Azimio/Nyamwezi 3 Arusha Arusha square service station Street 4 Arusha Five Star Service Station Arusha Arumeru Arusha Municipality plot No. 77 Block 'EE' 5 Mount Meru Petroleum Ltd. m1 Arusha Arusha Ngarenaro Arusha Municipality Kipelepele Anderson Mbuka - 6 Arusha Ngorongoro Loliondo Loliondo Arusha Mount Meru Petroleum Limited - 7 Arusha Arusha Levolosi, Mianzini M3 Mount Meru Petroleum Limited - M- Plot no. 1/S, Sokoine 8 Arusha Arusha 5 Road 9 Mount Meru Petroleum Ltd - Meru 7 Arusha Arusha Themi Hill, Njiro Plot No.368, Block 10 Mount Meru Petroleum Ltd - Meru 6 Arusha Arusha "DD", Sombetini area Sokoine Road/ 11 Mount Meru Petroleum Ltd - Meru 8 Arusha Arusha Shoprite Supermarket 12 Master Card Total Filling Station Arusha Arusha Themi - Sombetini 13 Master Card Total Filling Station Arusha Arusha Oljoro Road Arusha 14 Bamprass Star Service station Arusha Mianziani municipality 15 BP West Meru Service Station Arusha Arumeru Patandi, Tengeru BP Great North Service Station- 16 Arusha Arusha Mianzini Mianzini Njake Enterprises & Oil Transport - 17 Arusha Arusha Mianzini Mianzini Petrol Station HASS Petroleum (T) - Meserani Plot No.2, Block "A", 18 Arusha Monduli Petrol Station Meserani HASS Petroleum (T) - Kikatiti Petrol 19 Arusha Arumeru Kikatiti Station Nahil Petrol Station- Gapoil 20 Arusha Arusha Kwa Ngulelo Tanzania Limited Plot No. -
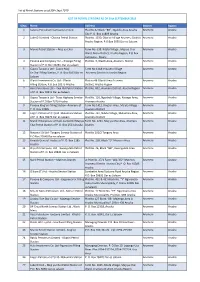
List of Petrol Stations As of 30Th Sept 2019 S/No. Name Address District
List of Petrol Stations as of 30th Sept 2019 LIST OF PETROL STATIONS AS OF 30th SEPTEMBER 2019 S/No. Name Address District Region 1 Saiteru Petroleum Company Limited Plot No.32 Block “BB”, Ngulelo Area Arusha Arumeru Arusha City P. O. Box 11809 Arusha 2 Lake Oil Limited - Olosiva Petrol Station Plot No. 1330, Olosiva Village Arumeru District Arumeru Arusha Arusha Region, P.O.Box 5055 Dar es Salaam 3 Munio Petrol Station – Maji ya Chai Farm No. 039, Kitefu Village , Maji ya Chai Arumeru Arusha Ward, Meru District, Arusha Region, P.O Box 160 Himo - Moshi 4 Panone and Company Ltd – Kisongo Filling Plot No. 3, Olasiti Area, Arumeru District Arumeru Arusha Station of P.O. Box 33285, Dar es salaam 5 Gapco Tanzania Ltd - Gapco Maji Farm No 1118 Imbaseni Village Arumeru Arusha Ya Chai Filling Station, P. O. Box 9103 Dar es Arumeru District in Arusha Region Salaam 6 Olasiti Investment Co. Ltd - Olasiti Plot no 46 Olasiti Area Arumeru Arumeru Arusha Filling Station, P.O.Box 10275 Arusha District, Arusha Region 7 Hass Petroleum Ltd – Kwa Idd Petrol Station Plot No. 832, Arumeru District, Arusha Region Arumeru Arusha of P. O. Box 78341 Dar es Salaam 8 Gapco Tanzania Ltd - Trans Highway Service Plot No. 116, Ngorbob Village, Kisongo Area, Arumeru Arusha Station of P.O Box 7370 Arusha Arumeru Arusha 9 Panone King’ori Filling Station- Arumeru of Farm No.1411, King’ori Area, Malula Village - Arumeru Arusha P. O. Box 33285 Arumeru District 10 Engen Petroleum (T) Ltd- Makumira Station Plot No. 797, Ndato Village, Makumira Area, Arumeru Arusha of P.