The Lesser Black-Backed Gull Larus Fuscus in England: How to Resolve a Conservation Conundrum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Herring Gull Complex (Larus Argentatus - Fuscus - Cachinnans) As a Model Group for Recent Holarctic Vertebrate Radiations
The Herring Gull Complex (Larus argentatus - fuscus - cachinnans) as a Model Group for Recent Holarctic Vertebrate Radiations Dorit Liebers-Helbig, Viviane Sternkopf, Andreas J. Helbig{, and Peter de Knijff Abstract Under what circumstances speciation in sexually reproducing animals can occur without geographical disjunction is still controversial. According to the ring species model, a reproductive barrier may arise through “isolation-by-distance” when peripheral populations of a species meet after expanding around some uninhabitable barrier. The classical example for this kind of speciation is the herring gull (Larus argentatus) complex with a circumpolar distribution in the northern hemisphere. An analysis of mitochondrial DNA variation among 21 gull taxa indicated that members of this complex differentiated largely in allopatry following multiple vicariance and long-distance colonization events, not primarily through “isolation-by-distance”. In a recent approach, we applied nuclear intron sequences and AFLP markers to be compared with the mitochondrial phylogeography. These markers served to reconstruct the overall phylogeny of the genus Larus and to test for the apparent biphyletic origin of two species (argentatus, hyperboreus) as well as the unex- pected position of L. marinus within this complex. All three taxa are members of the herring gull radiation but experienced, to a different degree, extensive mitochon- drial introgression through hybridization. The discrepancies between the mitochon- drial gene tree and the taxon phylogeny based on nuclear markers are illustrated. 1 Introduction Ernst Mayr (1942), based on earlier ideas of Stegmann (1934) and Geyr (1938), proposed that reproductive isolation may evolve in a single species through D. Liebers-Helbig (*) and V. Sternkopf Deutsches Meeresmuseum, Katharinenberg 14-20, 18439 Stralsund, Germany e-mail: [email protected] P. -

The Eiderling and Me Sailed O’Er O’Er Sailed Me and Eiderling the Very Best Shipmate
Explore, Spot things & Search for clues on the way the on clues for Search & things Spot Explore, 1 Easy to follow Map & Story & Map follow to Easy 2 3 The Eiderling “Well, Darwin, we are very lucky here at He started to comb the beach with his As he sat watching and listening the seals, or sea pigs as Fuzz called South Walney as we don’t get disturbed long beak, finding little snacks in the carefully a little caterpillar crawled them, hauling themselves up onto the Under the light of a May Darwin’s mum looked a little shocked. by many folks, so it’s a great place for sand. Darwin watched and then had a onto his shoulder. “Ahoy there, matey,” beach and felt very tiny in comparison. moon four little eider chicks “Well, my darling, you might need to you to watch the other creatures and go himself. “Are we hunting for pirate said the little hairy creature. “Need a “They can hold their breath for 20 explore the seven lands of Walney first start to understand them a little. Watch treasure?” asked Darwin. “Peep peep,” shipmate?” Darwin stared at his new pal minutes, I’ve been told.” made their way down to … just before you set sail.” how they move and talk to each other … said the oystercatcher and flew off out and nodded with a big, beaky smile. “My the shingle beach of Walney it’s a bit like cracking a secret code! Why to sea. Darwin smiled to himself and name’s Master Fuzz and I be pleased Darwin watched in wonder as the seals Island … one little eiderling “I really want to, Mum, but I’m scared don’t you start with someone who lives carried on exploring Shingle Beach. -

The Primary Moult in Four Gull Species Near Amsterdam J. Walters
THE PRIMARY MOULT IN FOUR GULL SPECIES NEAR AMSTERDAM J. WALTERS Received IS April 1978 CONTENTS I. Introduction and study area ... 32 2. Methods and materials ..... 33 2.1. Moult observations on dead gulls 33 2.2. Collection of newly shed primaries 34 2.3. Identification of primaries collected 34 2.4. "Average" primary per date of collection 36 2.5. Average date of shedding per primary 36 2.6. Sampling ......... 37 3. Results ............ 37 3.1. Black-headed Gull, adult .. 37 3.2. Black-headed Gull, sub-adult 38 3.3. Common Gull, adult .. 39 3.4. Common Gull, sub-adult .. 40 3.5. Herring Gull, adult 40 3.6. Herring Gull, sub-adult ... 42 3.7. Great Black-backed Gull, adult 42 3.8. Primary moult and reproduction 42 4. Discussion 43 5. Acknowledgements 46 6. Summary .. 46 7. References . 46 8. Samenvatting ... 46 1. INTRODUCTION AND STUDY AREA This paper deals with the primary moult in Black-headed Gull Larus ridibundus, Common Gull Larus canus, Herring Gull Larus argentatus and Great Black-backed Gull Larus marinus in areas near Amsterdam. Though a few studies on the moult of gulls already exist for the NW-European area, e.g. Stresemann & Stresemann (1966), Stresemann (1971), Barth (1975), Harris (1971), Ingolfsson (1970) and Verbeek (1977), such a study has not been published for a Dutch area and not for four Larus species at the same time and in the same restricted region. Moreover, for the three first mentioned species new information could be obtained on the timing of moult in relation to the incubation period. -

Walney Island
Cumbria Coastal Strategy Technical Appraisal Report for Policy Area 11c14 Walney Island (Technical report by Jacobs) CUMBRIA COASTAL STRATEGY - POLICY AREA 11C14 WALNEY ISLAND Policy area: 11c14 Walney Island Figure 1 Sub Cell 11c Arnside to Hodbarrow Point Location Plan of policy units. Baseline mapping © Ordnance Survey: licence number 100026791. 1 CUMBRIA COASTAL STRATEGY - POLICY AREA 11C14 WALNEY ISLAND 1 Introduction 1.1 Location and site description Policy units: 11c14.1 South End Hawes to Biggar (east side) 11c14.2 Biggar to Lenny Hill (east side) 11c14.3 South End Hawes to Hare Hill (open coast) 11c14.4 Hare Hill to Hillock Whins 11c14.5 Hillock Whins to Nanny Point Scar 11c14.6 Nanny Point Scar to Mill Scar 11c14.7 Mill Scar to north of West Shore Park 11c14.8 North Walney – from north of West Shore Park to Lenny Hill (both coasts) Responsibilities: Barrow Council Cumbria Country Council Private landowners Location: The policy area covers the entire frontage of Walney Island, both open coast (west coast) and channel (east coast) shorelines. Site Overview: Walney Island is composed of glacial deposits overlaying a rock platform; cliffs along the open coast are cut into these glacial sand and gravels, which provide little resistance to erosion. The two shorelines of Walney Island experience very different exposure conditions; the west coast is exposed to the Irish Sea whilst, in contrast, the east coast is sheltered from wave action. At either end of the Island, large sand and shingle spits extend towards the Duddon Estuary to the north and Morecambe Bay to the south. -

Field Identification of West Palearctic Gulls P
British Birds VOLUME 71 NUMBER 4 APRIL 1978 Field identification of west Palearctic gulls P. J. Grant The west Palearctic list includes 23 species of gulls: more than half the world total. Field guides—because of their concise format—provide inad equate coverage of identification and ageing, which has probably fostered the indifference felt by many bird watchers towards gulls. This five- part series aims to change that attitude nterest in identifying gulls is growing, as part of the recent general I improvement in identification standards, but doubtless also stimulated by the addition to the British and Irish list of no less than three Nearctic species in little over a decade (Laughing Gull Larus atricilla in 1966, Franklin's Gull L. pipixcan in 1970 and Ring-billed Gull L. delaivarensis in 1973). The realisation is slowly dawning that regular checking through flocks of gulls can be worthwhile. Just as important as identification is the ability to recognise the age of individual immatures. This is obviously necessary in studies of popula tion, distribution and migration, but is also a challenge in its own right to the serious bird-identifier. Indeed, identification and ageing go hand- in-hand, for it is only by practising his recognition skills on the common species—of all ages—that an observer will acquire the degree of familiarity necessary for the confident identification of the occasional rarity. The enormous debt owed to D.J. Dwight's The Gulls of the World (1925) is readily acknowledged. That work, however, has long been out of print and its format was designed for the museum and taxonomic worker; the present series of papers will provide a reference more suited to field observers. -

Have We All Missed the Point About Seagulls? Written by Joe Reynolds, Save Coastal Wildlife, Published: 20 February 2020
Have We All Missed the Point About Seagulls? Written by Joe Reynolds, Save Coastal Wildlife, Published: 20 February 2020 Along the picturesque Jersey Shore, a remarkable drama plays out almost every time someone visits a beach. No matter the season, from summer to spring, people will encounter gulls, erroneously known as seagulls. For me, I have a soft spot in my heart for these largely grey-and-white birds. They can be awe-inspiring sea creatures when soaring over the open ocean and dropping down out for the sapphire sky to catch a slimy fish or crusty crab. The sight of them brings to mind a sense of the long soft sand shorelines and sweeping winds and waves over a sun-and-shelled filled beach. Gulls are extraordinary birds. They are able to fly long distances and glide over the open ocean for hours in search of food. Gulls can fly as fast as 28 mph. They can even drink salty ocean water when thirsty. The birds have evolved to have a special pair of glands right above their eyes to flush the salt from their body through openings in their bill. This enables a gull to spend several days foraging for food atop salty ocean waters without needing to return to land just to get a drink of freshwater. James Gorman in 2019 wrote an article in The New York Times entitled: “In Defense of Sea Gulls: They’re Smart, and They Co-Parent, 50/50 All the Way.” He interviewed ornithologist Christopher Elphick from the University of Connecticut who also has a soft spot for gulls. -

Port of Barrow Operations and Maintenance Facility Scoping Opinion
Scoping Opinion Harbours Act 1964 Title: Port of Barrow – West Coast Operations and Maintenance Facility Applicant: DONG Energy Power (UK) Limited and Associated British Ports MMO Reference: DC10142 Page 1 of 15 Contents 1. Proposal Page 4 1.1 Project Background Page 4 2. Location Page 5 3. Environmental Impact Assessment (EIA) Page 6 4. EIA Scoping opinion Page 6 5. Nature conservation designations Page 7 5.1 European Marine Sites Page 7 5.2 Special Protection Areas Page 7 5.3 Special Areas of Conservation Page 9 5.4 Ramsar Page 9 5.5 Sites of Special Scientific Interest Page 9 5.6 Marine Conservation Zones Page 10 6. Coastal Processes Page 10 7. Fish Ecology and Fisheries Page 10 7.1 Fish Ecology Page 10 7.2 Fisheries Page 11 8. Archaeology Page 11 9. Navigation / Other Users of the Sea Page 12 Page 2 of 15 10. Water Quality Page 12 11. Waste and Disposal Page 13 12. Habitats Regulation Assessment Page 13 13. Cumulative Impacts Page 13 14. Additional Comments Page 14 14.1 Coordinates Page 14 14.2 Grey Seal Species Count Page 14 14.3 Decommissioning Page 14 14.4 Alternatives Page 14 14.5 Consultation Page 14 15. Conclusion Page 14 Page 3 of 15 1. Proposal DONG Energy Power (UK) Limited (“DE”) and Associated British Ports (“ABP”) propose to construct an Operations and Maintenance (“O&M”) facility at the Port of Barrow. The West Coast O&M facility will support three Offshore Windfarms (“OWF”): the existing Walney OWF (“WOW01+02”); the existing Barrow OWF (“BOW”) and the Walney Extension OWF (“WOW03+04 OWF”), which is currently under construction with completion due in 2018. -
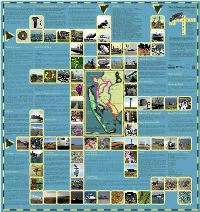
X FINAL ISLANDS of BARROW MAP PHOTO SIDE COLOURWAY 2 Copy
Prehistoric Islands An Industrial Revolution Barrow Airships Key Dates Prehistoric nds inc. axe heads have been discovered around the Islands of The expansion of Barrow-in-Furness was due to three men: Lord Cavendish, 7th 1911 Britains rst rigid airship HMA 1 ‘Mayy’, built in Barrow’s Cavendish 1127 Furness Abbey is established; The First Savignac Monastery in England The Islands of Barrow Barrow, many on Walney Island and Sandscale Haws. The coast oered stone age Duke of Devonshire (the nancier), Henry Schneider (local iron ore magnate) & Dock. 1134-1342 Furness Abbey becomes 2nd most powerful Cistercian Abbey in England communities, a wide range of foods and materials, often gathered during the James Ramsden (managing director of the Furness Railway Company). 1487 Lambert Simnal Lands on Piel Island & Claims English Throne hard winter months. It also oered opportunities for trade & communication via HMA 1 Mayy (named as such because “she may y” famously broke in two 1839 Henry Schneider a speculator & dealer in iron arrives during a test ight over Cavendish Dock but important lessons were learnt. sea-borne trac. Indeed, for much of prehistory, the sea was a link to the wider Ramsden built a ne house (now demolished) in Abbots wood above Furness Abbey. 1843 Only 32 dwellings & two pubs in the Hamlet of Barrow Later designs for rigid & non rigid airships were built by H.B. Pratt & Barnes world, rather than a barrier to it. Perhaps it is no surprise that the earliest cereal Some of Ramsden’s possessions & furniture were given to the Town Hall. Ramsden’s 1846 Furness Railway built by Schneider & James Ramsden to transport iron ore & slate Pictorial Wallis for the Vickers Airship Dept. -

Template for Submission of Scientific Information to Describe Areas Meeting Scientific Criteria for Ecologically Or Biologically Significant Marine Areas
Template for Submission of Scientific Information to Describe Areas Meeting Scientific Criteria for Ecologically or Biologically Significant Marine Areas Title/Name of the area: Ukrainian bays Abstract (in less than 150 words) Area located in the northern coast of the Black Sea, that encompasses 2 marine Important Bird and Biodiversity Areas mostly designated for the importance for several species of gulls, terns and seaducks. More than 1% of the global/biogeographic population of 10 seabird species occur in the area. The area encompasses also the non-breeding distribution of two Vulnerable seabirds – the Velvet Scoter Melanitta fusca and the Horned Grebe Podiceps auritus. Introduction Area located in the northern coast of the Black Sea (Figure 1), in the shallow waters (< 100 m deep) of the Ukranian bays of Yagorlyts'ka, Tendrivs'ka, Karkinits'ka and Dzharylgats'ka. The area includes 2 marine Important Bird and Biodiversity Areas (Figure 1), mostly designated for the importance for several species of gulls, terns and seaducks. More than 1% of the global/biogeographic population of 10 seabird species occur in the area (Greater Scaup Aythya marila, Great White Pelican Pelecanus onocrotalus, Great Cormorant Phalacrocorax carbo, Caspian Gull Larus cachinnans, Little Gull Hydrocoloeus minutus, Slender-billed Gull Larus genei, Mediterranean Gull Larus melanocephalus, Common Tern Sterna hirundo, Sandwich Tern Thalasseus sandvicensis and Caspian Tern Hydroprogne caspia: BirdLife International 2017a, 2017b). The area encompasses also the non- breeding distribution of two Vulnerable seabirds – the Velvet Scoter Melanitta fusca and the Horned Grebe Podiceps auritus (BirdLife International 2017c; Figure 2). Location The area is located in the North coast of Black Sea (ca. -

Laridaerefspart1 V1.2.Pdf
Introduction This is the first of two Gull Reference lists. It includes all those species of Gull that are not included in the genus Larus. I have endeavoured to keep typos, errors, omissions etc in this list to a minimum, however when you find more I would be grateful if you could mail the details during 2014 & 2015 to: [email protected]. Grateful thanks to Wietze Janse (http://picasaweb.google.nl/wietze.janse) and Dick Coombes for the cover images. All images © the photographers. Joe Hobbs Index The general order of species follows the International Ornithologists' Union World Bird List (Gill, F. & Donsker, D. (eds.) 2014. IOC World Bird List. Available from: http://www.worldbirdnames.org/ [version 4.2 accessed April 2014]). Cover Main image: Mediterranean Gull. Hellegatsplaten, South Holland, Netherlands. 30th April 2010. Picture by Wietze Janse. Vignette: Ivory Gull. Baltimore Harbour, Co. Cork, Ireland. 4th March 2009. Picture by Richard H. Coombes. Version Version 1.2 (August 2014). Species Page No. Andean Gull [Chroicocephalus serranus] 19 Audouin's Gull [Ichthyaetus audouinii] 37 Black-billed Gull [Chroicocephalus bulleri] 19 Black-headed Gull [Chroicocephalus ridibundus] 21 Black-legged Kittiwake [Rissa tridactyla] 6 Bonaparte's Gull [Chroicocephalus philadelphia] 16 Brown-headed Gull [Chroicocephalus brunnicephalus] 20 Brown-hooded Gull [Chroicocephalus maculipennis] 20 Dolphin Gull [Leucophaeus scoresbii] 31 Franklin's Gull [Leucophaeus pipixcan] 34 Great Black-headed Gull [Ichthyaetus ichthyaetus] 41 Grey Gull [Leucophaeus -

Identification of the Larus Canus Complex
Identification of the Larus canus complex Peter Adriaens & Chris Gibbins ecent decades have seen a wealth of new in- brachyrhynchus) in western North America (for- Rformation published on the identification and merly, the name Mew Gull was often reserved for taxonomy of the so-called ‘large white-headed the Nearctic taxon only). Treatment of these four gulls’. In contrast, the smaller taxa have been rath- taxa in recent literature is often minimal, and er neglected. This is certainly the case with Mew some information is even misleading; for instance, Gull Larus canus, a familiar species that occurs in the two most recent gull monographs heinei is throughout the Northern Hemisphere but whose pictured as a bird that is more or less inseparable geographical variation and field identification re- from canus (Olsen & Larsson 2003, Howell & main poorly understood. Despite its wide distri- Dunn 2007). This hinders identification in various bution, Mew Gull has been treated as a single places around the world; eg, how should we ap- species, consisting of four subspecies: nominate proach the identification of a vagrant kamtschat- L c canus (Common Gull; hereafter canus) in schensis when we do not know what heinei looks Europe (including parts of European Russia), L c like? In some ways, heinei is a key taxon but one heinei (Russian Common Gull; hereafter heinei) that has remained something of a mystery. Another throughout Russia, including large parts of Siberia, problem is that variation in nominate canus is still L c kamtschatschensis (Kamchatka Gull; hereafter not widely understood and is sometimes under- kamtschatschensis) in eastern Siberia, and L c estimated. -

Mytern Booklet
MyTern AUSTRALIA A po ck et g ui de t o th e te rn s of A us tr al ia Caspian Terns, Glenn Ehmke MyTern.indd 1 22/5/19 10:44 am Co nt en ts Introduction ......................................................... 3 General habitat .....................................................4 Nesting habitat ..................................................... 5 Chicks ................................................................. 6 Feeding strategies ................................................. 7 Threats ............................................................... 8 Tips for identifying similar-looking terns.................... 9 !"#$%#&R(")*[,#&(--------------------------------------------------(./ 0#)1&(%1(\%345(-----------------------------------------------------(67 8*1%5*)%13(5#)1&---------------------------------------------------9/ Counting techniques ............................................ 32 BirdLife Australia projects .....................................38 Ke y fo r sp ec ie s’ p ro fil es bp = breeding plumage nbp = non-breeding plumage juv = juvenile plumage Note: the size (cm) of each species refers to body length, the images are not drawn to scale, and the distribution maps are based on all species’ records in Australia (from BirdLife Australia Atlas data). 2 MyTern.indd 2 22/5/19 10:44 am In tr od uc ti on 04%&(:**;,#5(%&(<([#,=($*>"<1%*1(?*)(>*1%5*)%13(@A&5)<,%<1( terns. MyTern contains key information about the habitat, =%&5)%:A5%*1B(1#&5%13()#CA%)#>#15&(<1=(%=#15%[$<5%*1(*?(5#)1&- Terns are in the subfamily Sterninae and are closely related to gulls, but are smaller, slimmer and longer-tailed. Noddies are very closely related and appear similar to terns (but have a wedge-shaped tail and inverse colouration: light cap and dark body). Twenty species of terns and three species of noddies have been recorded in Australia, including migratory &"#$%#&( <1=( D<3)<15&-( 8*&5( 5#)1&( 4A15( [&4( *)( <)54)*"*=&( (insects and crustaceans) by diving, skimming the surface of the water, or on the wing.