IDENTIFICATION of VULNERABLE SPECIES INCIDENTALLY CAUGHT in MEDITERRANEAN FISHERIES Seabirds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Order CHARADRIIFORMES: Waders, Gulls and Terns Suborder LARI
Text extracted from Gill B.J.; Bell, B.D.; Chambers, G.K.; Medway, D.G.; Palma, R.L.; Scofield, R.P.; Tennyson, A.J.D.; Worthy, T.H. 2010. Checklist of the birds of New Zealand, Norfolk and Macquarie Islands, and the Ross Dependency, Antarctica. 4th edition. Wellington, Te Papa Press and Ornithological Society of New Zealand. Pages 191, 223 & 227-228. Order CHARADRIIFORMES: Waders, Gulls and Terns The family sequence of Christidis & Boles (1994), who adopted that of Sibley et al. (1988) and Sibley & Monroe (1990), is followed here. Suborder LARI: Skuas, Gulls, Terns and Skimmers Condon (1975) and Checklist Committee (1990) recognised three subfamilies within the Laridae (Larinae, Sterninae and Megalopterinae) but this division has not been widely adopted. We follow Gochfeld & Burger (1996) in recognising gulls in one family (Laridae) and terns and noddies in another (Sternidae). The sequence of species for Stercorariidae and Laridae follows Peters (1934) and for Sternidae follows Bridge et al. (2005). Family LARIDAE Rafinesque: Gulls Laridia Rafinesque, 1815: Analyse de la Nature: 72 – Type genus Larus Linnaeus, 1758. Genus Larus Linnaeus Larus Linnaeus, 1758: Syst. Nat., 10th edition 1: 136 – Type species (by subsequent designation) Larus marinus Linnaeus. Gavia Boie, 1822: Isis von Oken, Heft 10: col. 563 – Type species (by subsequent designation) Larus ridibundus Linnaeus. Junior homonym of Gavia Moehring, 1758. Hydrocoleus Kaup, 1829: Skizz. Entw.-Gesch. Eur. Thierw.: 113 – Type species (by subsequent designation) Larus minutus Linnaeus. Chroicocephalus Eyton, 1836: Cat. Brit. Birds: 53 – Type species (by monotypy) Larus cucullatus Reichenbach = Larus pipixcan Wagler. Gelastes Bonaparte, 1853: Journ. für Ornith. -

Morphological Variation Among Herring Gulls (Larus Argentatus) and Great Black-Backed Gulls (Larus Marinus) in Eastern North America Gregory J
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by University of New England University of New England DUNE: DigitalUNE Environmental Studies Faculty Publications Environmental Studies Department 4-2016 Morphological Variation Among Herring Gulls (Larus Argentatus) And Great Black-Backed Gulls (Larus Marinus) In Eastern North America Gregory J. Robertson Environment Canada Sheena Roul Environment Canada Karel A. Allard Environment Canada Cynthia Pekarik Environment Canada Raphael A. Lavoie Queen's University See next page for additional authors Follow this and additional works at: http://dune.une.edu/env_facpubs Part of the Ornithology Commons Recommended Citation Robertson, Gregory J.; Roul, Sheena; Allard, Karel A.; Pekarik, Cynthia; Lavoie, Raphael A.; Ellis, Julie C.; Perlut, Noah G.; Diamond, Antony W.; Benjamin, Nikki; Ronconi, Robert A.; Gilliland, Scott .;G and Veitch, Brian G., "Morphological Variation Among Herring Gulls (Larus Argentatus) And Great Black-Backed Gulls (Larus Marinus) In Eastern North America" (2016). Environmental Studies Faculty Publications. 22. http://dune.une.edu/env_facpubs/22 This Article is brought to you for free and open access by the Environmental Studies Department at DUNE: DigitalUNE. It has been accepted for inclusion in Environmental Studies Faculty Publications by an authorized administrator of DUNE: DigitalUNE. For more information, please contact [email protected]. Authors Gregory J. Robertson, Sheena Roul, Karel A. Allard, Cynthia Pekarik, Raphael A. Lavoie, Julie C. Ellis, Noah G. Perlut, Antony W. Diamond, Nikki Benjamin, Robert A. Ronconi, Scott .G Gilliland, and Brian G. Veitch This article is available at DUNE: DigitalUNE: http://dune.une.edu/env_facpubs/22 Morphological Variation Among Herring Gulls (Larus argentatus) and Great Black-Backed Gulls (Larus marinus) in Eastern North America Author(s): Gregory J. -

Sabine's Gull Large Caspian Or Birdwatchers White- Larophiles Armenian Or Headed Heuglin’S Gulls Gull?!!!!
گردآوری: حمید جبّاری اسفند 96 ﻻروفایل دیوانگان در پی کاکایی!! Sabine's Gull Large Caspian or Birdwatchers white- Larophiles Armenian or headed Heuglin’s Gulls Gull?!!!! کاکایی ارمنی تفاوت در فرم شکل و منقار کاکایی خزری کاکایی سیبری پشت سیاه کوچک ارمنی خزری کاکایی خزری کاکایی پشت سیاه کوچک کاکایی ارمنی کاکایی سیبری کاکایی پشت سیاه کوچک کاکایی پشت سیاه کوچک کاکایی خزری کاکایی ارمنی کاکایی خزری کاکایی ارمنی Armenian Gull – Larus armenicus Main ID features at rest Main ID features of adult in flight . Bill – rather short, shorter than fuscus . Wing – adults show dark grey upperparts, Gonys – medium size, but might look confusingly black on primaries usually to P5 and large at short range, and in juvenile males. Legs – from pink in 1st winter to yellow in adult mirrors on P10 only, nevertheless about . 20% of the individuals may show black up Eye – dark in most individuals, up to 10% will show to P4 and 10% will even show some black pale eye to some extend, but all pale eyed on P3. individuals will have dark spots on the iris . Same works for mirror on P9 . Size –larger than fuscus, but size can vary from 1st - 2nd winter birds show very pale very small females to very large males . upperwing, especially median coverts and Head Color – head typically very inner primaries rounded, closer to heuglini Armenian Gull – Larus armenicus, adult spring, Armenian Gull – Larus armenicus, adult winter Armenian Gull – Larus armenicus, adult Armenian Gull – Larus armenicus, adult winter Armenian Gull – Larus armenicus, 1st summer birds Armenian Gull – Larus armenicus, 1st winter Armenian Gull – Larus armenicus, 1st summer Armenian Gull – Larus armenicus, advanced 2nd winter, second cycle Armenian Gull The bill may be already largely yellow at this age. -

Tringa Ery-Dend Syr.P65 139 19/10/2004, 16:53
Birds in Europe – Gulls and terns Country Breeding pop. size (pairs) Year(s) Trend Mag.% References Larus cachinnans Albania 90 – 110 96–02 0 0–19 YELLOW-LEGGED GULL Austria 10 – 25 98–02 + >80 Azerbaijan 10,000 – 15,000 96–00 (0) (0–19) Non-SPECE (1994: —) Status Secure Belarus 150 – 400 97–02 + 10–19 Criteria — Belgium 2–2 00–02 +N1 Bosnia & HG Present 90–03 ? – European IUCN Red List Category — Bulgaria 5,000 – 7,000 96–02 + 0–19 Criteria — Croatia (25,000 – 50,000) 02 (–) (0–19) 16 Cyprus (100 – 200) 98–02 (+) (0–19) Global IUCN Red List Category — Czech Rep. 0–5 00 +N Criteria — France 40,000 – 45,000 97–00 + 50–79 1 Georgia 300 – 1,000 94–02 – 20–29 Larus cachinnans is a widespread breeder in coastal areas of southern and eastern Germany 79 – 89 95–99 + 50–79 Greece (3,000 – 5,000) 95–00 (+) (0–19) Europe, which constitutes >50% of its global breeding range. Its European breeding Hungary 3 – 9 95–02 (F) (–) 14,7 population is large (>310,000 pairs), and increased between 1970–1990. Although Italy 40,000 – 50,000 03 + 30–49 Macedonia 50 – 250 90–00 (F) (–) 7 there were declines in Croatia and Georgia during 1990–2000, populations across Malta 150 – 180 90–02 + 0–19 1 the rest of its European range increased or were stable, and the species showed a Moldova 0 – 30 90–00 F 20–29 marked increase overall. Consequently, it is evaluated as Secure. Netherlands 16 – 32 98–00 ? – 1 Poland 150 – 230 97–02 +N2 Portugal 20,000 – 30,000 02 (+) (–) 2,5 Azores Present 02 ? – Madeira (5,000 – 10,000) 02 (0) (0–19) No. -

Non-Living (Abiotic) Elements Shape Habitat
Forest Birds Fast Facts LIGHT: Dominated by tall trees, layered canopy = very shady, openings made from a fallen tree provide sunny areas AIR: Trees slow wind, except along forest edges and openings. Shade = cool temps WATER: Fog and rain collect in tree branches and drip to ground. SOIL: Lots of organics (needles, decaying leaves, tree trunks) makes lots of space for air and water. Soil absorbs and holds water like a sponge. Varied Thrush • Slender bill good at gleaning soft foods like insects, pillbugs, snails, worms, fruits and some seeds from ground. • Hops and pauses to look for food. Flips leaves and debris with bill. • Perching feet – three toes forward, hind toe back. • Can sing 2 separate notes at same time and breathe while singing. Common Raven Varied Thrush • Versatile beak. Eats everything from carrion and garbage, to eggs, nestlings, insects, seeds, rodents and fruit. • Strong, sturdy feet and grasping toes to manipulate food and perch. • Acrobatic flight, hops on ground. Uses sight to find food. Northern Flicker • The toes are placed two forward, two back to grip firmly, while the tail feathers are stiff and pointed to help brace while pounding. • Bill is shaped like a chisel. Flickers don’t excavate as much as other types of woodpeckers so have a slightly curved bill and less sharp. Common Raven • Flickers eat insects and are especially fond of ants (flickers will forage on the ground as well as on trees). Probe and explore crevices. • Distinctive flight – flap, dip, flap, dip • Woodpeckers have long, sticky and barbed tongues to extract bugs. -
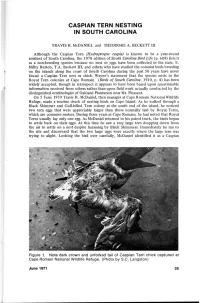
Caspian Tern Nesting in South Carolina
CASPIAN TERN NESTING IN SOUTH CAROLINA TRAVIS H. McDANIEL and THEODORE A. BECKETT III Although the Caspian Tern (Hydroprogne caspia) is known to be a year-round resident of South Carolina, the 1970 edition of South Carolina Bird Life (p. 608) lists it as a non-breeding species because no nest or eggs have been collected in the state. E. Milby Burton, T.A. Beckett III, and others who have studied the colonial birds breeding on the islands along the coast of South Carolina during the past 50 years have never found a Caspian Tern nest or chick. Wayne's statement that the species nests in the Royal Tern colonies at Cape Romain (Birds of South Carolina, 1910, p. 4) has been widely accepted, though in retrospect it appears to have been based upon questionable information received from others rather than upon field work actually conducted by the distinguished ornithologist of Oakland Plantation near Mt. Pleasant. On 5 June 1970 Travis H. McDaniel, then manager at Cape Romain National Wildlife Refuge, made a routine check of nesting birds on Cape Island. As he walked through a Black Skimmer and Gull-billed Tern colony at the south end of the island, he noticed two tern eggs that were appreciably larger than those normally laid by Royal Terns, which are common nesters. During three years at Cape Romain, he had noted that Royal Terns usually lay only one egg. As McDaniel returned to his patrol truck, the birds began to settle back on their eggs. At this time he saw a very large tern dropping down from the air to settle on a nest despite harassing by Black Skimmers. -

Avon Bird Report 2008
AVON BIRD REPORT 2008 AVON ORNITHOLOGICAL GROUP Front cover: Great Crested Grebe. Photograph by Richard Andrews. Rear cover: Map of the Avon area computer generated by S. Godden, Dept. of Geography, University of Bristol. Text drawings by R.M. Andrews, J.P. Martin, R.J. Prytherch, B.E. Slade, the late L.A. Tucker and Anon. Typeset in WORD 2007 and printed by Healeys, Ipswich ISSN Number – 0956-5744 2 Avon Bird Report 2008 CONTENTS BTO advert Front cover Avon Ornithological Group (AOG) Front cover Editorial H.E. Rose 3 A guide to the records required by the Avon Bird Report 4 Species and subspecies for which descriptions are required 5 A review of 2008 R.J. Higgins 7 Weather in 2008 R.L. Bland 11 Migrant date summary 14 Introduction to systematic list 15 Contributors of records 18 Systematic list Swans and geese R. Mielcarek 19 Ducks M.S. Ponsford 23 Game birds R. Mielcarek 36 Divers to Spoonbill R.J. Higgins 38 Raptors B. Lancastle 45 Water Rail to Crane R. Mielcarek 53 Waders H.E. Rose 56 Skuas to Auks R.M. Andrews 71 Doves to Woodpeckers R. Mielcarek 83 Passerines, Larks to Dipper J. P. Martin 91 Passerines, Wren to Buntings R.L. Bland 97 Escaped, released and hybrid birds R Mielcarek 126 Birds of the Downs, 1994 - 2008 R.L. Bland 127 Metal pollution in Bristol: An assessment using bird of prey S. M. Murgatroyd 137 feathers Bitterns breeding at Chew Valley Lake 1997 - 2001 K. E. Vinicombe 143 Black-necked Grebes breeding at Chew Valley Lake in 1998 K. -

The Herring Gull Complex (Larus Argentatus - Fuscus - Cachinnans) As a Model Group for Recent Holarctic Vertebrate Radiations
The Herring Gull Complex (Larus argentatus - fuscus - cachinnans) as a Model Group for Recent Holarctic Vertebrate Radiations Dorit Liebers-Helbig, Viviane Sternkopf, Andreas J. Helbig{, and Peter de Knijff Abstract Under what circumstances speciation in sexually reproducing animals can occur without geographical disjunction is still controversial. According to the ring species model, a reproductive barrier may arise through “isolation-by-distance” when peripheral populations of a species meet after expanding around some uninhabitable barrier. The classical example for this kind of speciation is the herring gull (Larus argentatus) complex with a circumpolar distribution in the northern hemisphere. An analysis of mitochondrial DNA variation among 21 gull taxa indicated that members of this complex differentiated largely in allopatry following multiple vicariance and long-distance colonization events, not primarily through “isolation-by-distance”. In a recent approach, we applied nuclear intron sequences and AFLP markers to be compared with the mitochondrial phylogeography. These markers served to reconstruct the overall phylogeny of the genus Larus and to test for the apparent biphyletic origin of two species (argentatus, hyperboreus) as well as the unex- pected position of L. marinus within this complex. All three taxa are members of the herring gull radiation but experienced, to a different degree, extensive mitochon- drial introgression through hybridization. The discrepancies between the mitochon- drial gene tree and the taxon phylogeny based on nuclear markers are illustrated. 1 Introduction Ernst Mayr (1942), based on earlier ideas of Stegmann (1934) and Geyr (1938), proposed that reproductive isolation may evolve in a single species through D. Liebers-Helbig (*) and V. Sternkopf Deutsches Meeresmuseum, Katharinenberg 14-20, 18439 Stralsund, Germany e-mail: [email protected] P. -

Ring Recoveries of Lesser Crested Tern Thalasseus Bengalensis Along the Maharashtra Coast, India Raju Kasambe & Vaibhav Deshmukh
88 Indian BirDS Vol. 7 No. 3 (Publ. 21 October 2011) another recent specimen recovered near Valparai in Tamil Nadu and Sri Lanka. 2nd ed. Delhi: Oxford University Press. (Robin & Rao 2006). Recent sight records for this decade are Ambedkar, V. C., 1981. Occurrence of the Sooty Tern (Sterna fuscata) from the Lakshadweep archipelago, and Kerala. Mike Prince in Bombay - an authentic record. J. Bombay Nat. Hist. Soc. 78 (2): records two individuals seen off Agatthi and Kavaratti Islands 377–378. Ambedkar, V. C., 1983. Occurrence of the Sooty Tern (Sterna fuscata) at in the Lakshadweep group of islands (Prince 2008). One was Point Calimere, Tamil Nadu. J. Bombay Nat. Hist. Soc. 80 (1): 215. found at Kallambalam, Kollam, and another at Kothamangalam, Baker, E. C. S., 1912. The Sooty Tern (Sterna fuliginosa) in Cachar. J. both in Kerala, at the end of May 2011 (Sreekumar 2011). A Bombay Nat. Hist. Soc. 21 (2): 684. flock was observed at sea off the coast near Kannur on 28 May Betts, F. N., 1939. The breeding of the Indian Sooty Tern (Sterna fuscata 2011 (Praveen 2011). infuscata) in the Laccadive Islands. J. Bombay Nat. Hist. Soc. 40 From the distribution map given in Kazmierczak (2000), (4): 763–764. there are only a few scattered records of the bird, mostly along Inglis, C. M., 1902. Occurrence of the Sooty Tern (Sterna fuliginosa) the western shores, and very few off the eastern seaboard. This in the Darbhanga district, Tirhut. J. Bombay Nat. Hist. Soc. 14 (3): 627–628. is consistent with the records of the species given above. -

European Red List of Birds 2015
Chlidonias niger (Black Tern) European Red List of Birds Supplementary Material The European Union (EU27) Red List assessments were based principally on the official data reported by EU Member States to the European Commission under Article 12 of the Birds Directive in 2013-14. For the European Red List assessments, similar data were sourced from BirdLife Partners and other collaborating experts in other European countries and territories. For more information, see BirdLife International (2015). Contents Reported national population sizes and trends p. 2 Trend maps of reported national population data p. 4 Sources of reported national population data p. 6 Species factsheet bibliography p. 10 Recommended citation BirdLife International (2015) European Red List of Birds. Luxembourg: Office for Official Publications of the European Communities. Further information http://www.birdlife.org/datazone/info/euroredlist http://www.birdlife.org/europe-and-central-asia/european-red-list-birds-0 http://www.iucnredlist.org/initiatives/europe http://ec.europa.eu/environment/nature/conservation/species/redlist/ Data requests and feedback To request access to these data in electronic format, provide new information, correct any errors or provide feedback, please email [email protected]. THE IUCN RED LIST OF THREATENED SPECIES™ BirdLife International (2015) European Red List of Birds Chlidonias niger (Black Tern) Table 1. Reported national breeding population size and trends in Europe1. Country (or Population estimate Short-term population trend4 Long-term population trend4 Subspecific population (where relevant) 2 territory) Size (pairs)3 Europe (%) Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Direction5 Magnitude (%)6 Year(s) Quality Albania 0-10 <1 2002-2012 poor 0 0 2002-2012 poor 0 0 1980-2012 poor Armenia Present <1 2002-2012 ? ? Belarus 6,000-22,000 11 2000-2012 medium F 25-267 2000-2012 medium F 25-267 1980-2012 medium Bosnia & HG 1-5 <1 2010-2014 poor ? ? Bulgaria 25-52 <1 2005-2012 medium - 5-20 2000-2012 poor - 10-30 1980-2012 poor Czech Rep. -

Iucn Red Data List Information on Species Listed On, and Covered by Cms Appendices
UNEP/CMS/ScC-SC4/Doc.8/Rev.1/Annex 1 ANNEX 1 IUCN RED DATA LIST INFORMATION ON SPECIES LISTED ON, AND COVERED BY CMS APPENDICES Content General Information ................................................................................................................................................................................................................................ 2 Species in Appendix I ............................................................................................................................................................................................................................... 3 Mammalia ............................................................................................................................................................................................................................................ 4 Aves ...................................................................................................................................................................................................................................................... 7 Reptilia ............................................................................................................................................................................................................................................... 12 Pisces ................................................................................................................................................................................................................................................. -

Laughing Gulls Breed Primarily Along the Pacific Coast of Mexico and the Atlantic and Caribbean Coasts from S
LAUGHING GULL Leucophaeus atricilla non-breeding visitor, regular winterer L.a. megalopterus Laughing Gulls breed primarily along the Pacific coast of Mexico and the Atlantic and Caribbean coasts from s. Canada to Venezuela, and they winter S to Peru and the Amazon delta (AOU 1998, Howell and Dunn 2007). It and Franklin's Gull were placed along with other gulls in the genus Larus until split by the AOU (2008). Vagrant Laughing Gulls have been reported in Europe (Cramp and Simmons 1983) and widely in the Pacific, from Clipperton I to Wake Atoll (Rauzon et al. 2008), Johnston Atoll (records of at least 14 individuals, 1964-2003), Palmyra, Baker, Kiribati, Pheonix, Marshall, Pitcairn, Gambier, and Samoan Is, as well as Australia/New Zealand (King 1967; Clapp and Sibley 1967; Sibley and McFarlane 1968; Pratt et al. 1987, 2010; Garrett 1987; Wragg 1994; Higgins and Davies 1996; Vanderwerf et al. 2004; Hayes et al. 2015; E 50:13 [identified as Franklin's Gull], 58:50). Another interesting record is of one photographed attending an observer rowing solo between San Francisco and Australia at 6.5° N, 155° W, about 1400 km S of Hawai'i I, 1-2 Nov 2007. They have been recorded almost annually as winter visitors to the Hawaiian Islands since the mid-1970s, numbers increasing from the NW to the SE, as would be expected of this N American species. The great majority of records involve first-year birds, and, despite the many records in the S Pacific, there is no evidence for a transient population through the Hawaiian Islands, or of individuals returning for consecutive winters after departing in spring.