Benicar (Olmesartan Medoxomil) Tablets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BENICAR HCT Tablets
® BENICAR HCT Tablets (OLMESARTAN MEDOXOMIL-HYDROCHLOROTHIAZIDE) WARNING: FETAL TOXICITY When pregnancy is detected, discontinue Benicar HCT as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus. See Warnings: Fetal Toxicity DESCRIPTION BENICAR HCT® (olmesartan medoxomil-hydrochlorothiazide) is a combination of an angiotensin II receptor antagonist (AT1 subtype), olmesartan medoxomil, and a thiazide diuretic, hydrochlorothiazide (HCTZ). Olmesartan medoxomil, a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan medoxomil is 2,3-dihydroxy-2-butenyl 4-(1-hydroxy-1-methylethyl)-2 propyl-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl]imidazole-5-carboxylate, cyclic 2,3 carbonate. Its empirical formula is C29H30N6O6 and its structural formula is: Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.6. It is practically insoluble in water and sparingly soluble in methanol. Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzo-thiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is: 1 Reference ID: 3227549 Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.7. Hydrochlorothiazide is slightly soluble in water but freely soluble in sodium hydroxide solution. BENICAR HCT® is available for oral administration in tablets containing 20 mg or 40 mg of olmesartan medoxomil combined with 12.5 mg of hydrochlorothiazide, or 40 mg of olmesartan medoxomil combined with 25 mg of hydrochlorothiazide. Inactive ingredients include: hydroxypropylcellulose, hypromellose, lactose, low-substituted hydroxypropylcellulose, magnesium stearate, microcrystalline cellulose, red iron oxide, talc, titanium dioxide and yellow iron oxide. -

Effects of Olmesartan Vs Irbesartan on Metabolic Parameters and Visfatin in Hypertensive Obese Women
European Review for Medical and Pharmacological Sciences 2010; 14: 759-763 Effects of olmesartan vs irbesartan on metabolic parameters and visfatin in hypertensive obese women D.A. DE LUIS, R. CONDE, M. GONZALEZ SAGRADO, R. ALLER, O. IZAOLA, J.L. PEREZ CASTRILLON, E. ROMERO, M.J. CASTRO Institute of Endocrinology and Nutrition, Medicine School and Unit of Investigation. Hospital Rio Hortega. RD-056/0013 RETICEF. University of Valladolid. Valladolid (Spain) Abstract. – Background: Angiotensin II reg- dence of this rising tide of obesity and associated ulates the production of adipokines. The objective pathologies has led, in the last years, to a dramat- was to study the effect of treatment with irbesartan versus olmesartan in obese hypertensive women. ic increase of researches on the role of adipose Subjects: A sample of 34 obese hypertensive tissue as an active participant in controlling the women was analyzed in a prospective way with a body’s physiology2. randomized trial. Patients were randomized to irbe- Visfatin was recently identified as a protein sartan (300 mg/day) or olmesartan (40 mg/day) for preferentially expressed in visceral adipose tis- 3 months. Weight, body mass index, blood pres- sue, compared with subcutaneous adipose tis- sure, basal glucose, insulin, total cholesterol, LDL- sue3. It can be found in skeletal muscle, liver, cholesterol, HDL-cholesterol, triglycerides, HOMA and visfatin were determined at basal time and af- bone marrow and lymphocytes, where it was ter 3 months of treatment. initially identified as pre-B-cell colony-enhanc- Results: Thirty four patients gave informed con- ing factor (PBEF). Fukuhara et al4 clearly sug- sent and were enrolled in the study. -
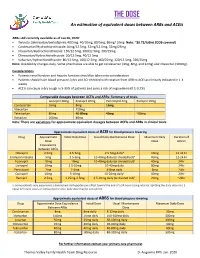
THE DOSE an Estimation of Equivalent Doses Between Arbs and Aceis
THE DOSE An estimation of equivalent doses between ARBs and ACEIs ARBs still currently available as of Jan 26, 2020: Twynsta (telmisartan/amlodipine): 40/5mg. 40/10mg, 80/5mg, 80mg/ 10mg Note: ~$0.73/tablet (ODB covered) Candesartan/Hydrochlorothiazide:16mg/12.5mg, 32mg/12.5mg, 32mg/25mg Irbesartan/Hydrochlorothiazide: 150/12.5mg, 300/12.5mg, 300/25mg Olmesartan/Hydrochlorothiaizde: 20/12.5mg, 40/12.5mg Valsartan/Hydrochlorothiazide: 80/12.5mg, 160/12.5mg, 160/25mg, 320/12.5mg, 320/25mg Note: Availability changes daily. Some pharmacies are able to get candesartan (4mg, 8mg, and 32mg) and irbesartan (300mg). Considerations Patients renal function and hepatic function should be taken into consideration Patients should have blood pressure, lytes and SCr checked with rotation from ARB to ACEI as clinically indicated in 1-4 weeks ACEIs can cause a dry cough in 5-35% of patients and carry a risk of angioedema (0.1-0.2%) Comparable dosages between ACEIs and ARBs- Summary of trials Lisinopril 20mg Enalapril 20mg Perindopril 4mg Ramipril 10mg Candesartan 16mg 8mg 16mg Irbesartan 150mg Telmisartan 80mg 40-80mg 40mg ~80mg Valsartan 160mg 80mg Note: There are variations for approximate equivalent dosages between ACEIs and ARBs in clinical trials. Approximate equivalent doses of ACEI for blood pressure lowering Drug Approximate Initial Daily Dose Usual Daily Maintenance Dose Maximum Daily Duration of Dose Dose Action Equivalence Between ACEIs Cilazapril 2.5mg 2.5-5mg 2.5-5mg dailya 10mg 12-24 hr Enalapril maleate 5mg 2.5-5mg 10-40mg daily (or divided bid)a 40mg 12-24 hr Fosinopril 10mg 10mg 10-40mg daily (or divided bid)a 40mg 24hr Lisinopril 10mg 2.5-10mg 10-40mg daily 80mg 24hr Perindopril 2mg 2-4mg 4-8mg daily 8mg 24hr Quinapril 10mg 5-10mg 10-20mg dailya 40mg 24hr Ramipril 2.5mg 1.25mg-2.5mg 2.5-10mg daily (or divided bid)a 20mg ~24hr a: Some patients may experience a diminished antihypertensive effect toward the end of a 24-hour dosing interval. -

Olmesartan Medoxomil 10 Mg Film-Coated Tablets Olmesartan Medoxomil 20 Mg Film-Coated Tablets Olmesartan Medoxomil 40 Mg Film-Coated Tablets Olmesartan Medoxomil
Package leaflet: Information for the user Olmesartan medoxomil 10 mg Film-coated Tablets Olmesartan medoxomil 20 mg Film-coated Tablets Olmesartan medoxomil 40 mg Film-coated Tablets olmesartan medoxomil Read all of this leaflet carefully before you start taking this medicine because it contains important information for you. - Keep this leaflet. You may need to read it again. - If you have any further questions, ask your doctor or pharmacist. - This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours. - If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4. What is in this leaflet 1. What Olmesartan medoxomil is and what it is used for 2. What you need to know before you take Olmesartan medoxomil 3. How to take Olmesartan medoxomil 4. Possible side effects 5. How to store Olmesartan medoxomil 6. Contents of the pack and other information 1. What Olmesartan medoxomil is and what it is used for Olmesartan medoxomil belongs to a group of medicines called angiotensin-II receptor antagonists. They lower blood pressure by relaxing the blood vessels. This medicine is used for the treatment of high blood pressure (also known as ‘hypertension’) in adults and in children and adolescents aged 6 to less than 18 years. High blood pressure can damage blood vessels in organs such as the heart, kidneys, brain and eyes. In some cases this may lead to a heart attack, heart or kidney failure, stroke or blindness. -

Comparison of Effects of Olmesartan and Telmisartan on Blood Pressure and Metabolic Parameters in Japanese Early-Stage Type-2 Diabetics with Hypertension
7 Hypertens Res Vol.31 (2008) No.1 p.7-13 Original Article Comparison of Effects of Olmesartan and Telmisartan on Blood Pressure and Metabolic Parameters in Japanese Early-Stage Type-2 Diabetics with Hypertension Shiho NAKAYAMA1), Hirotaka WATADA1), Tomoya MITA1), Fuki IKEDA1), Tomoaki SHIMIZU1), Hiroshi UCHINO1), Yoshio FUJITANI1),2), Takahisa HIROSE1),2), and Ryuzo KAWAMORI1),2) Angiotensin II type-1 receptor blockers (ARBs) are regarded as first-line treatments for type-2 diabetes with hypertension. Despite the availability of various types of ARBs, there are no comparative studies of their effects on patients with diabetes. In this open-label prospective crossover study, we compared the effects of olmesartan (20 mg/day) and telmisartan (40 mg/day). Twenty Japanese early-stage type-2 diabetes patients with hypertension treated with valsartan (80 mg/day) for at least 8 weeks were recruited to this study. At study entry, valsartan was changed to olmesartan (20 mg/day) or telmisartan (40 mg/day) and administered for 8 weeks. The drugs were then switched and treatment was continued for another 8 weeks. We analyzed the blood pressure lowering effects of each drug by 24-h ambulatory blood pressure monitor- ing at 0, 8, and 16 weeks. Simultaneously, we measured metabolic parameters and inflammation markers. Olmesartan lowered mean systolic and diastolic blood pressure more significantly than did telmisartan. While there were no differences between the groups in metabolic parameters, including HbA1c and adi- ponectin, the decreases in serum interleukin-6 and highly sensitive C-reactive protein were more significant by olmesartan treatment. Our results indicate that olmesartan has more potent arterial blood pressure low- ering and anti-inflammatory effects than telmisartan. -

Product Information for Olmetec (Olmesartan Medoxomil)
Attachment 1: Product information for AusPAR Olmetec Merck Sharp & Dohme (Australia) Pty Ltd PM-2011-01941-3-3 Final 26 February 2013. This Product Information was approved at the time this AusPAR was published. APPROVED PRODUCT INFORMATION OLMETEC (olmesartan medoxomil) NAME OF THE MEDICINE OLMETEC (olmesartan medoxomil), a prodrug, is hydrolyzed to olmesartan during absorption from the gastrointestinal tract. Olmesartan is a selective AT1 subtype angiotensin II receptor antagonist. Olmesartan medoxomil (CAS no. 144689-63-4) is described chemically as 2,3-dihydroxy-2- butenyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[p-(o-1H-tetrazol-5-ylphenyl)benzyl] imidazole-5-carboxylate, cyclic 2,3-carbonate. Its empirical formula is C29H30N6O6 and its structural formula is: DESCRIPTION Olmesartan medoxomil is a white to light yellowish-white powder or crystalline powder with a molecular weight of 558.59. It is practically insoluble in water and sparingly soluble in methanol. OLMETEC is available for oral use as film-coated tablets containing 10 mg, 20 mg, or 40 mg olmesartan medoxomil. OLMETEC tablets also contain the following inactive ingredients: microcrystalline cellulose, low-substituted hydroxypropylcellulose, lactose, hydroxypropylcellulose, magnesium stearate, and Opadry OY-S-38956 that contains titanium dioxide, talc, and hydroxypropylmethylcellulose. OLMETEC extemporaneous suspension contains additional inactive ingredients: purified water, Ora-Sweet® (syrup vehicle) and Ora-Plus® (suspending vehicle). Ora-Sweet® contains citric acid, flavouring, glycerine, methylparaben, potassium sorbate, sodium phosphate, sorbitol, sucrose, and purified water. Ora-Plus® contains calcium sulphate, carrageenan, citric acid, dimethicone antifoam emulsion, methylparaben, microcrystalline cellulose, sodium carboxymethylcellulose, potassium sorbate, sodium phosphate monobasic, trisodium phosphate, xanthan gum, and purified water. -

Comparison of the Effects of Telmisartan and Olmesartan On
921 Hypertens Res Vol.31 (2008) No.5 p.921-929 Original Article Comparison of the Effects of Telmisartan and Olmesartan on Home Blood Pressure, Glucose, and Lipid Profiles in Patients with Hypertension, Chronic Heart Failure, and Metabolic Syndrome Tatsuya SASAKI1), Yoshiki NODA1), Yoshinori YASUOKA1), Hiroaki IRINO1), Haruhiko ABE1), Hidenori ADACHI1), Susumu HATTORI1), Hirokazu KITADA1), Daisuke MORISAWA1), and Kunio MIYATAKE1) We compared the effects of telmisartan and olmesartan in 20 patients with chronic heart failure and meta- bolic syndrome. The subjects underwent once-daily 40 mg telmisartan for at least 3 months before switching to once-daily 20 mg olmesartan for the next 3 months (post 1). They were then treated with 3 months of once-daily 40 mg telmisartan (post 2). Systolic and diastolic blood pressure in the early morning, plasma B- type natriuretic peptide, serum total cholesterol, low-density lipoprotein cholesterol, and triglyceride levels were increased at post 1 (p<0.005, p<0.05, p<0.05, p<0.05, p<0.05, and p<0.005 vs. baseline, respectively) before returning to their baseline values at post 2. The changes in plasma B-type natriuretic peptide levels correlated significantly with the shifts in systolic and diastolic blood pressure in the early morning at posts 1 and 2. Meanwhile, there were no fluctuations in either blood pressure in the late evening or in the outpa- tient room; nor were there fluctuations in heart rate. Simultaneously, neither serum high-density lipoprotein cholesterol nor fasting blood sugar levels differed significantly between posts. Moreover, telmisartan had more beneficial effects on glucose and lipid profiles in patients with relatively high HbA1c, serum total and low-density lipoprotein cholesterol, and triglyceride levels. -

Arbs Or Aceis, That Is the Question
837 Hypertens Res Vol.29 (2006) No.11 p.837-838 Editorial Comment ARBs or ACEIs, That Is the Question Tomoaki ISHIGAMI1), Kazuaki UCHINO1), and Satoshi UMEMURA1) (Hypertens Res 2006; 29: 837–838) Key Words: angiotensin converting enzyme 2, angiotensin-(1-7), renin angiotensin system, angiotensin con- verting enzyme inhibitor, angiotensin II receptor blocker The renin-angiotensin system (RAS) plays a pivotal role in evidence that olmesartan, one of the most potent ARBs, the maintenance of body fluid, electrolyte homeostasis and exerts a pharmacological action to lower blood pressure by cardiovascular functions. It is widely accepted that its impair- modifying the Ang-(1-7)/ACE2 system. They examined the ments in cardiovascular tissue both locally and systemically Ang-(1-7)/ACE2 system during chronic olmesartan treatment cause progressive cardiovascular remodeling, and ultimately of stroke-prone spontaneously hypertensive rat (SHR-SP), a result in morbidity and mortality for human beings. There- rodent model of hypertension. They found that olmesartan fore, disruptions to this system by angiotensin converting treatment increased ACE2 expression in the kidney and heart, enzyme inhibitors (ACEIs) and angiotensin II receptor block- and enhanced the action of Ang-(1-7), using Ang-(1-7) antag- ers (ARBs) are currently utilized as one of the major medical onist with restoring Ang II elevation in vivo. They speculated treatment options available among physicians. However, that ACE inhibition by elevated Ang-(1-7) leads to Ang II emerging evidences indicate that angiotensin (1-7) (Ang-(1- suppression, which is recognized as a unique feature of olme- 7)) is an endogenous counteracting peptide against RAS and sartan treatment (8). -

Comparative Proteinuria Management of Different Angiotensin-Converting Enzyme Inhibitors Or Angiotensin Receptor Blockers for No
Comparative proteinuria management of different angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for normotensive patients with CKD: a Bayesian network meta-analysis Huizhen Ye*, Zhihao Huo*, Peiyi Ye, Guanqing Xiao, Zhe Zhang, Chao Xie and Yaozhong Kong Nephrology Department, The First People's Foshan Hospital, Foshan, Guangdong, China * These authors contributed equally to this work. ABSTRACT Background. Both angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) are blood pressure-lowering agents, but they are also being used to control proteinuria in early chronic kidney disease (CKD) patients. However, clinically, some patients present merely proteinuria without hypertension. No guidelines pointed out how to select treatments for proteinuria in normotensive patients. Thus, we conducted a Bayesian network analysis to evaluate the relative effects of different kinds of ACEI or ARB or their combination on proteinuria and blood pressure reduction. Methods. The protocol was registered in PROSPERO with ID CRD42017073721. A comprehensive literature database query was carried out systematically according to PICOS strategies. The primary outcome was reduction in proteinuria, and the secondary outcomes were eGFR reduction and blood pressure reduction. Random- effects pairwise and Bayesian network meta-analyses were used to estimate the effect of different regimens. Results. A total of 14 RCTs with 1,098 patients were included in the analysis. All Submitted 2 September 2019 treatment strategies of ACEI, ARB or their combination had significantly greater efficacy Accepted 16 January 2020 Published 12 March 2020 in reducing proteinuria than placebo in normotensive CKD patients. The combination therapy of olmesartan+temocapril had the highest probability (22%) of being the most Corresponding author Yaozhong Kong, [email protected] effective treatment to reduce proteinuria in normotensive CKD patients. -

Angiotensin II Receptor Antagonists (Arbs), Renin
Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy Program Summary This program applies to Commercial, GenPlus, NetResults A series, Blue Partner, SourceRx, and Health Insurance Marketplace formularies. OBJECTIVE The intent of the Angiotensin II Receptor Antagonists (ARBs), Renin Inhibitors, and Combinations Step Therapy (ST) program is to encourage use of cost-effective generic products - ACEIs, ACEI combinations (ACEI/diuretics or ACEI/calcium channel blockers [CCBs]), ARBs, or ARB combinations - over the more expensive brand ARBs, brand ARB combinations, brand renin inhibitors and renin inhibitor combinations (renin inhibitor/diuretic, renin inhibitor/ARB, or renin inhibitor/CCB). This program will accommodate for use of brand products when generic prerequisites cannot be used due to previous trial and failure, documented intolerance, FDA labeled contraindication, or hypersensitivity. Requests for brand ARB or renin inhibitor products will be reviewed when patient-specific documentation is provided. TARGET AGENTS Angiotensin II Receptor Antagonists (ARBs), Combinations Brand Generic Atacand® candesartana Atacand HCT® candesartan/HCTZab Avapro® irbesartana Avalide® irbesartan/HCTZab Azor® olmesartan/amlodipinea Benicar® olmesartana Benicar HCT® olmesartan/HCTZab Byvalson™ nebivolol/valsartan Cozaar® losartana Diovan® valsartana Diovan HCT® valsartan/HCTZab Edarbi® azilsartan Edarbyclor® azilsartan/chlorthalidone Eprosartan eprosartan Exforge® valsartan/amlodipinea Exforge HCT® valsartan/amlodipine/HCTZab -

Beta Blocker and Blood Pressure Medication Guidelines
Beta Blocker and Blood Pressure Medication Guidelines Beta Blockers Please take this medication as normally scheduled, even on the morning of surgery with a small sip of water, unless otherwise instructed by your surgeon. Acebutolol Bystolic InnoPran XL Metoprolol Tartrate/HTZ Propranolol/HTZ Toprol Atenolol Carteolol Kerlone Nadolol Sectral Toprol XL Atenolol/chlorthalidone Cartrol Labetalol Nadolol/bendroflumethiazide Sorine Trandate Betagan Carvediolol Levatol Nebivolol Sotalol Trandate HCL Betapace Coreg Levobetaxolol Nebivolol HCL Sotalol HCL Visken Betapace AF Corgard Levobunolol Nebivolol Hydrochloride Tenoretic Zebeta Betaxolol Corzide Lopressor Normodyne Tenormin Ziac Bisoprolol Esmolol Lopressor HCT Penbutolol Tenormin IV Bisoprolol/fumurate Inderal Lopressor HTZ Pindolol Timolide Bisoprolol/HTZ Inderal LA Metapranolol Propranolol Timolol Blocardren Inderide Metoprolol Propranolol HCL Timolol Maleate/HTZ Brevibloc Inderide LA Metoprolol/HTZ Propranolol Hydrochloride Timolol/ HTZ Ace Inhibitors (ACEI) Please DO NOT take this medication on the morning of surgery unless otherwise instructed by your surgeon. Accupril Enalapril maleate/diltiazem Lotensin HCT Prinzide Uniretic Accuretic Enalapril maleate/HCT Lotrel Quinapril Univasc Aceon Enalapril diltiazem Mavik Quinapril HCL Vaseretic Altace Enalapril/felodipine Moexipril Quinapril HCL/HCT Vasotec Benazepril Enalapril/HCT Moexipril HCL Quinapril HCT Zestoretic Benazepril/amlodipine Enalaprilat Moexipril HCL/HCT Quinaretic Zestril Benazepril HCL Fosinopril Moexipril HCT Ramipril -

Effects of Telmisartan Vs Olmesartan on Metabolic Parameters, Insulin Resistance and Adipocytokines in Hypertensive Obese Patients
Nutr Hosp. 2010;25(2):275-279 ISSN 0212-1611 • CODEN NUHOEQ S.V.R. 318 Original Effects of telmisartan vs olmesartan on metabolic parameters, insulin resistance and adipocytokines in hypertensive obese patients D. A. de Luis, R. Conde, M. González-Sagrado, R. Aller, O. Izaola, A. Dueñas, J. L. Pérez Castrillón and E. Romero Institute of Endocrinology and Nutrition. Medicine School and Unit of Investigation. Hospital Río Hortega. RD-056/0013 RETICEF. University of Valladolid. Valladolid. Spain. Abstract EFECTOS DE TELMISARTAN VS OLMESARTAN SOBRE PARÁMETROS ANTROPOMÉTRICOS, Background: Angiotensin II regulates the production RESISTENCIA A LA INSULINA of adipokines. The objective was to study the effect of Y ADIPOCITOQUINAS EN PACIENTES treatment with telmisartan versus olmesartan in hyper- HIPERTENSOS OBESOS tensive obese and overweight patients. Subjects: A sample of 65 overweight and obese patients with mild to moderate hypertension was analyzed in a Resumen prospective way with a randomized trial. Patients were randomized to telmisartan (80 mg/day) or olmesartan (40 Introducción: La angiotensina II puede regular la pro- mg/day) for 3 months. Weight, body mass index, blood ducción de adipocitoquinas. El objetivo de nuestro tra- pressure, basal glucose, insulin, total cholesterol, LDL- bajo fue evalaur el efecto sobre parámetros bioquímicos cholesterol, HDL-cholesterol, triglycerides, HOMA, del tratamiento con telmisartan versus olmesartan en QUICKI, leptin and adiponectin were determined at pacientes obesos hipertensos. basal time and after 3 months of treatment. Pacientes: Se analizó una muestra de 65 pacientes con Results: Sixty five patients gave informed consent and hipertensión moderada severa y obesidad, mediante un were enrolled in the study.