Microbial Features Indicating the Recovery of Soil Ecosystem Strongly Affected by Mining and Ore Processing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps
ISSN 0532-0488 WORLD SOIL RESOURCES REPORTS 106 World reference base for soil resources 2014 International soil classification system for naming soils and creating legends for soil maps Update 2015 Cover photographs (left to right): Ekranic Technosol – Austria (©Erika Michéli) Reductaquic Cryosol – Russia (©Maria Gerasimova) Ferralic Nitisol – Australia (©Ben Harms) Pellic Vertisol – Bulgaria (©Erika Michéli) Albic Podzol – Czech Republic (©Erika Michéli) Hypercalcic Kastanozem – Mexico (©Carlos Cruz Gaistardo) Stagnic Luvisol – South Africa (©Márta Fuchs) Copies of FAO publications can be requested from: SALES AND MARKETING GROUP Information Division Food and Agriculture Organization of the United Nations Viale delle Terme di Caracalla 00100 Rome, Italy E-mail: [email protected] Fax: (+39) 06 57053360 Web site: http://www.fao.org WORLD SOIL World reference base RESOURCES REPORTS for soil resources 2014 106 International soil classification system for naming soils and creating legends for soil maps Update 2015 FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2015 The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. -

Redalyc.Quaternary Pedostratigraphy of the Nevado De Toluca Volcano
Revista Mexicana de Ciencias Geológicas ISSN: 1026-8774 [email protected] Universidad Nacional Autónoma de México México Solleiro Rebolledo, Elizabeth; Macías, José Luis; Gama Castro, Jorge Enrique; Sedov, Sergey; Sulerzhitsky, Leopold D. Quaternary pedostratigraphy of the Nevado de Toluca volcano Revista Mexicana de Ciencias Geológicas, vol. 21, núm. 1, 2004, pp. 101-109 Universidad Nacional Autónoma de México Querétaro, México Available in: http://www.redalyc.org/articulo.oa?id=57221112 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista Mexicana de Ciencias Geológicas,Quaternary v. 21 pedostratigraphy núm. 1, 2004, p. of101-109 the Nevado de Toluca volcano 101 Quaternary pedostratigraphy of the Nevado de Toluca volcano Elizabeth Solleiro-Rebolledo1,*, José Luis Macías2, Jorge Gama-Castro1, Sergey Sedov1, and Leopold D. Sulerzhitsky3 1 Instituto de Geología, 2 Insituto de Geofísica, Universidad Nacional Autónoma de México, Cd. Universitaria, 04510 México, D.F., Mexico 3 Geological Institute, Russian Academy of Sciences,Pyzhevsky 7, 119017 Moscow, Russia * [email protected] ABSTRACT Paleosol sequences of the Nevado de Toluca volcano (NTV) preserve a detailed record of late Quaternary environmental change. In fact, some studies have been used the NTV paleosols to interpret paleoenvironmental conditions, however some uncertainties still remain concerning their stratigraphic position and correlation. In this paper, we present the results of the NTV pedostratigraphy by using different paleosol units and based on present day tephrostratigraphy. We recognized eight Pleistocene paleosols, labeled PT0–PT7 which cover the 100,000–10,000 yr BP interval. -

Guidebook for Field Excursions Xllth International Symposium and Field Seminar on Paleopedology
'1 ■ I VJ 1 ■ ’ »■ " ' . ... !■ 1|Щ 11 ■ Institute of Geography, Russian Academy of Sciences Moscow State University, Soil Science Institute V.V. Dokuchaev Soil Science Institute Institute of History and Material Culture, Russian Academy of Sciences Kursk State University Upetsk State University W. Alekhin Centrul-Chemozemic State Biospheric Reserve Natural Architectural-Archaeological Museum-Resort "Dhmogorid' Guidebook for Field Excursions Xllth International Symposium and Field Seminar on Paleopedology ‘Paleosols, pedosediments and landscape morphology as archives of environmental evolution” 10 -15 August, 2013, Kursk, Russia Moscow 2013 ■ ....... ... - Guidebook for Field Excursions Xllth International Symposium and Field Seminar on Paleopedology CONTENTS Introduction (S.A. Sycheva) 5 1. General characterization of the environment and soils of the Central Russian Upland (S.A. Sycheva, I. V. Kovda, A. V.Kashkin) 9 1.1. Geology and relief 9 1.2. Climate 10 1.3. Hydrology 11 1.4. Vegetation 11 1.5. Soils 12 1.6. Human history and agricultural development of landscapes 12 KURSK SITE 14 2. Aleksandrov Quarry (S.A. Sycheva, E. D. Sheremetskaya, T.M. Grigorieva, M.A. Bronnikova, S.N. Sedov, V.S. Gunova, A.N. Siniakova, P.R.Pushkina) 14 2.1. General characteristics, geochronology and stratigraphy 14 2.2. Description of the composite generalized stratigraphic section 15 2.2.1. Morphology 15 2.2.2. Analytical data 20 2.3. Ryshkov (Mikulino) paleosols and paleocatena (MIS 5e) 23 2.3.1. Ryshkov paleosol on the paleoslope (section 15): interpretation of properties 23 2.3.2. Ryshkov pedolithocomplex in the bottom 27 2.4. Early Valdai Kukuev and Streletsk paleosols (MIS 5 & MIS 5a) 28 2.4.1. -

Tigray Esia Soil Characteristics
TIGRAY ESIA SOIL CHARACTERISTICS INTRODUCTION The purpose of this Chapter is to describe the receiving environment in terms of soils within the Project site and surrounding area, to establish the extent to which agricultural soils will be removed from the site as a result of the proposed Tigray IAIP and RTC site developments and to identify potential risks to the identified soils and recommend associated mitigation measures. This was established by undertaking a fertility analysis of the soils sampled at the sites and calculating the potential risks that the proposed development will pose to the soils, with and without mitigation measures being put in place. The description provided in this chapter is based on primary data obtained from site investigations. AIMS AND OBJECTIVES The main aims of the soils investigation within this overarching study are threefold: 1. To establish the agricultural value of the soils to be removed in order for the proposed Tigray IAIP and its associated RTC to be built; 2. To establish the main soil-associated risks identified as a result of the proposed project, and 3. To identify potential mitigation measures associated with these risks. METHODOLOGY DESKTOP ASSESSMENT A detailed desktop assessment was undertaken for the Tigray IAIP and RTC sites. This included assessing the ISRIC World Soils Database, based on the World Reference Base Classification System (WRB, 2006) in order to establish the soil types according to an international standard system. Information associated with the outcomes of the ISRIC world soils database outcomes was reviewed, as was general information associated with Ethiopian soils, and, specifically, those found in the Tigray region. -

Chemical Properties of the Forest Litter in Istria and the Croatian Littoral
Ekológia (Bratislava) Vol. 33, No. 3, p. 242–251, 2014 doi:10.2478/eko-2014-0023 CHEMICAL PROPERTIES OF THE FOREST LITTER IN ISTRIA AND THE CROATIAN LITTORAL ANDRIJA ŠPOLJAR1, DAMIR BARČIĆ2*, TOMISLAVA PEREMIN VOLF1, STJEPAN HUSNJAK3, IVICA MARTINOVIĆ1 1 College of Agriculture at Križevci, Milislava Demerca 1, 48260 Križevci, Croatia 2 University of Zagreb Faculty of Forestry, Svetošimunska 25, 10 000 Zagreb, Croatia; e-mail: [email protected] 3 University of Zagreb Faculty of Agriculture, Svetošimunska 25, 10 000 Zagreb, Croatia * Author for correspondence Abstract Špoljar A., Barčić D., Peremin Volf T., Husnjak S., Martinović I.: Chemical properties of the forest litter in Istria and the Croatian Littoral. Ekológia (Bratislava), Vol. 33, No. 3, p. 242–251, 2014. The experiment was set up in the forest ecosystem with diverse vegetation zones in the area of Istria and the Croatian Littoral. Research included the following systematic soil units: lithic lepto- sols, rendzic leptosols, rendzic leptosols – eroded, mollic leptosols, chromic cambisol and chromic luvisols. The average quantity of the forest litter in the studied systematic soil units reaches 13.36 t/ha (Tables 1−3). The “wealth” of organic matter in the studied soil units can be presented with the following series: chromic cambisols (CMx) > mollic leptosols (LPm), organogenic, rendzic leptosols (LPk) > lithic leptosols (LPq) > chromic cambisols (CMx) – Terra rossa, chromic luvi- sols (LVx) > rendzic leptosols (LPk) – eroded. As expected, the lowest value of total nitrogen was found in the lithic leptosols in relation to almost all the other soils, except when compared with chromic cambisol and rendzic leptosols (p < 0.05). -

Annex: Soil Groups, Characteristics, Distribution and Ecosystem Services
Status of the World’s Main Report Soil Resources Annex Soil groups, characteristics, distribution and ecosystem services © FAO | Giuseppe Bizzarri © FAO INTERGOVERNMENTAL TECHNICAL PANEL ON SOILS Disclaimer and copyright Recommended citation: FAO and ITPS. 2015. Status of the World’s Soil Resources (SWSR) – Main Report. Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, Rome, Italy The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO. ISBN 978-92-5-109004-6 © FAO, 2015 FAO encourages the use, reproduction and dissemination of material in this information product. Except where otherwise indicated, material may be copied, downloaded and printed for private study, research and teaching purposes, or for use in non-commercial products or services, provided that appropriate acknowledgement of FAO as the source and copyright holder is given and that FAO’s endorsement of users’ views, products or services is not implied in any way. -
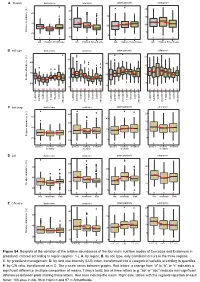
Figure S4. Boxplots of the Variation of the Relative Abundances of the Four
A Region bacterivore omnivore plant parasite eukaryvore ● ● ● 20 40 ● ● ● 60 ● ● ● ● ● 40 15 ● 30 ● ● ● ● 40 ● ● ● 10 ● ● 20 ● ● ● 20 Relative abundance (%) 5 20 0 b a b 10 c b a a a a a a a Alb Hainich Schorfheide Alb Hainich Schorfheide Alb Hainich Schorfheide Alb Hainich Schorfheide B soil type bacterivore omnivore plant parasite eukaryvore ● ● ● ● ● 20 40 ● ● ● ● ● ● 60 ● ● ● ● 40 ● 15 ● ● ● ● 30 ● ● ● ● ● ● ● ● ● ● ● ● ● ● 40 ● ● ● ● ● ● ● 20 10 ● ● ● ● ● ● ● ● ● 20 ● ● Relative abundance (%) ● ● 20 5 0 10 b ac ab a bc bc ab ab b b ab ab a a ab ab a a a a a a a a a a a a a a a a Luvisol Luvisol Luvisol Luvisol Vertisol Vertisol Vertisol Vertisol Gleysol Gleysol Gleysol Gleysol Histosol Histosol Histosol Histosol Leptosol Leptosol Leptosol Leptosol Cambisol Cambisol Cambisol Cambisol Stagnosol Stagnosol Stagnosol Stagnosol Albeluvisol Albeluvisol Albeluvisol Albeluvisol C land usage bacterivore omnivore plant parasite eukaryvore ● ● ● ● ● 40 20 ● ● 60 ● ● ● ● ● 40 ● ● 15 ● 30 ● ● 40 ● ● ● ● ● 20 ● ● 10 ● ● 20 ● Relative abundance (%) 20 5 10 0 a a a a a a b a a a a a pasture mowed meadow pasture mowed meadow pasture mowed meadow pasture mowed meadow meadow meadow meadow meadow D LUI bacterivore omnivore plant parasite eukaryvore ● ● ● ● ● 40 ● ● ● ● ● 20 60 ● ● ● ● 40 ● ● ● ● 30 ● 15 ● ● ● ● 40 ● ● ● ● 20 ● ● ● 20 ● 10 Relative abundance (%) ● 20 ● ● 10 0 ● b a b b a a a c b 5 b a a ● low moderate high low moderate high low moderate high low moderate high E C/N ratio bacterivore omnivore plant parasite eukaryvore ● ● ● 20 ● 40 ● ● ● ● 60 ● ● 40 15 ● ● ● ● ● ● 30 ● ● ● ● 40 ● 10 ● ● ● ● 20 ● ● ● 20 Relative abundance (%) 5 20 a a a 10 b a a 0 a b b b a a - >10 - 15 ~10 7 - <10 >10 - 15 ~10 7 - <10 >10 - 15 ~10 7 - <10 >10 - 15 ~10 7 - <10 Figure S4. -

Classification of Soil Systems on the Basis of Transfer Factors of Radionuclides from Soil to Reference Plants
IAEA-TECDOC-1497 Classification of soil systems on the basis of transfer factors of radionuclides from soil to reference plants Proceedings of a final research coordination meeting organized by the Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture and held in Chania, Crete, 22–26 September 2003 June 2006 IAEA-TECDOC-1497 Classification of soil systems on the basis of transfer factors of radionuclides from soil to reference plants Report of the final research coordination meeting organized by the Joint FAO/IAEA Programme of Nuclear Techniques in Food and Agriculture held in Chania, Crete, 22–26 September 2003 June 2006 The originating Section of this publication in the IAEA was: Food and Environmental Protection Section International Atomic Energy Agency Wagramer Strasse 5 P.O. Box 100 A-1400 Vienna, Austria CLASSIFICATION OF SOIL SYSTEMS ON THE BASIS OF TRANSFER FACTORS OF RADIONUCLIDES FROM SOIL TO REFERENCE PLANTS IAEA, VIENNA, 2006 IAEA-TECDOC-1497 ISBN 92–0–105906–X ISSN 1011–4289 © IAEA, 2006 Printed by the IAEA in Austria June 2006 FOREWORD The IAEA Basic Safety Standards for Radiation Protection include the general requirement to keep all doses as low as reasonably achievable, taking account of economic and social considerations, within the overall constraint of individual dose limits. National and Regional authorities have to set release limits for radioactive effluent and also to establish contingency plans to deal with an uncontrolled release following an accident or terrorist activity. It is normal practice to assess radiation doses to man by means of radiological assessment models. In this context the IAEA published (1994), in cooperation with the International Union of Radioecologists (IUR), a Handbook of Parameter Values for the Prediction of Radionuclide Transfer in Temperate Environments to facilitate such calculations. -

Impact of Land Use and Geological Conditions on Selected Physical Soil
160 JOZEF VARGA, RADOSLAVA KANIANSKA, JÁN SPIŠIAKSOIL SCIENCE ANNUAL Vol. 69 No. 3/2018: 160–168 DOI: 10.2478/ssa-2018-0016 JOZEF VARGA*, RADOSLAVA KANIANSKA, JÁN SPIŠIAK Matej Bel University Banská Bystrica, Faculty of Natural Sciences Tajovského 40, 974 01 Banská Bystrica, Slovakia Impact of land use and geological conditions on selected physical soil properties in relation to the earthworm abundance and biomass along an altitudinal gradient in Slovakia Abstract: The aim of the study was to analyse the impact of land use and altitudinal gradient including geological conditions on selected soil physical properties with subsequent effect on earthworms as important soil organisms. The research was conducted at three study sites (Oèová – OC, Tajov – TA, Liptovská Teplièka – LT) situated in the different climatic and natural conditions of Slovakia each with 3 plots differing in land use (arable land - AL, permanent grasslands – PG, forest land – FL). During 2014 over two periods, we measured soil penetration resistance (PR) with total depth of the measurement (DP) and soil moisture (SM). Earth- worms were hand sorted counted and weighed. We found out high variability of measured parameters conditioned by time, space (altitudinal gradient) and land use. PR values of all measurements ranged from 0.19 to 5.00 MPa, DP values from 0.02 to 0.80 m and soil moisture from 2 to 50%. Paired samples test confirmed differences between different land use types mainly between AL and FL plots. There were confirmed significant differences between three ecological gradients in all observed properties with one exception. Correlations among observed variables under different altitudinal gradients and land use types were found. -

Labile Forms of Carbon and Soil Aggregates
Soil & Water Res., 11, 2016 (4): 00–00 Original Paper doi: 10.17221/182/2015-SWR Labile Forms of Carbon and Soil Aggregates Erika TOBIAŠOVÁ1, Gabriela BARANČÍKOVÁ2, Erika GÖMÖRYOVÁ3, Jarmila MAKOVNÍKOVÁ2, Rastislav SKALSKÝ 2, Ján HALAS 2, Štefan KOCO 4, Zuzana TARASOVIČOVÁ2, Jozef TAKÁČ 2 and Miroslav ŠPAŇO1 1Department of Soil Science, Faculty of Agrobiology and Food Resources, Slovak University of Agriculture, Nitra, Slovak Republic; 2Soil Science and Conservation Research Institute – National Agriculture and Food Centre, Bratislava, Slovak Republic; 3Faculty of Forestry, Technical University in Zvolen, Zvolen, Slovak Republic; 4Department of Geography and Applied Geoinformatics, University of Presov, Prešov, Slovak Republic Abstract Tobiašová E., Barančíková G., Gömöryová E., Makovníková J., Skalský R., Halas J., Koco Š., Tarasovičová Z., Takáč J., Špaňo M. (2016): Labile forms of carbon and soil aggregates. Soil & Water Res. Soil organic matter (SOM) plays an important role in the soil aggregation and vice versa, its incorporation into the soil aggregates is one of the mechanisms of soil organic carbon stabilization. In this study the influence of labile carbon fractions on the fractions of dry-sieved (DSA) and wet-sieved (WSA) macro-aggregates and the relationship between the content of total organic carbon (TOC) and its labile fractions in the soil and in the fractions of macro-aggregates were determined. The experiment included six soil types (Eutric Fluvisol, Mol- lic Fluvisol, Haplic Chernozem, Haplic Luvisol, Eutric Cambisol, -

ISRIC Soil Reference Collection Management Plan
ISRIC soil reference collection management plan Reference soils serving global issues ISRIC Report (Draft for discussion) Stephan Mantel September 2017 ISRIC – World Soil Information Contents 1. ISRIC world reference soils collection ........................................................ 5 1.1 History and criteria reference collection ................................................. 5 1.2 Gaps in reference collection ................................................................. 6 1.4 Criteria for increasing value of soil reference collection ............................ 7 1.4.1 Taxonomic gaps in reference collection ............................................. 7 1.4.2 Geographical representation............................................................ 8 1.4.3 Special environments ..................................................................... 9 1.4.4 scientific and educational interest .................................................... 9 1.4.5 framework for selection of sites and soils ........................................ 13 2. Soil sampling for reference collections ..................................................... 14 2.1 Protocol collection of new reference soil profiles and samples ............. 14 3. Provisional list of sites and soils for potential future sampling ..................... 16 4. Concluding remarks .............................................................................. 18 5. References........................................................................................... 19 1. ISRIC -

The Effect of Layered Lithology on Soil Formation and Landscape Evolution a Case Study in the Flint Hills, Kansas, USA
The effect of layered lithology on soil formation and landscape evolution A case study in the Flint Hills, Kansas, USA Marte Stoorvogel March, 2019 The effect of layered lithology on soil formation and landscape evolution A case study in the Flint Hills, Kansas, USA M.M. (Marte) Stoorvogel 951113808060 Master thesis Earth and Environment: Soil Geography and Earth Surface Dynamics March, 2019 Wageningen, The Netherlands Soil Geography and Landscape group Wageningen UR Supervisors: W.M. (Marijn) van der Meij MSc Soil Geography and Landscape group Wageningen UR, Netherlands Dr. A.J.A.M (Arnaud) Temme Department of Geography Kansas State University, USA ii Abstract Soil-landscape relations originate from interactions between pedogenic and geomorphic processes. These relations can especially be strong in systems with a layered bedrock lithology as a result of the strong influence of parent material on soil formation. However, previous research has not yet focused on feedbacks between soil and landscape evolution in layered bedrock systems. Therefore, the objective of this research was to get a better understanding of the influence of lithology on soil formation and landscape evolution across hillslopes on layered bedrock. This study was conducted at the Konza Prairie ecological research station in Kansas, USA, where layers of limestone and shale alternate. A field survey, laboratory analysis and model study were performed to reach the objective. Results show that soil properties vary strongly across hillslopes on layered bedrock and that differences in elevation, slope steepness, profile curvature and parent material affect these properties. Especially soil depth, surface stoniness and vegetation coverage follow a clear pattern across the hillslopes, with thin soils, high surface stoniness and low vegetation coverage at convex positions and steep slopes.