Laboratory Studies of Organic Material Under Simulated Martian Conditions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cata Alogue E 68
Grosvenor Prints 19 Shelton Street Covent Garden London WC2H 9JN Tel: 020 7836 1979 Fax: 020 7379 6695 E-mail: [email protected] www.grosvenorprints.com Dealers in Antique Prints & Books Catalogue 68 February Listing Item 369: The Grand Master or Adventures of Qui Hi? in Hindostan All items listed are illustrated on our web site: www.grosvenorprints.com Registered in England No. 1305630 Registered Office: 2, Castle Business Villlage, Station Roaad, Hampton, Middlesex. TW12 2BX. Rainbrook Ltd. Directors: N.C. Talbot. T.D.M. Rayment. C.E. Elliis. E&OE VAT No. 217 6907 49 Stock: 43657 4. [Wax and Candle Invoice.] Bo.t of W. & J. Barton, Wax & Tallow Chandlers, No. 26 Bishopsgate with opposite Threadneedle Street. [1834.] Engraved invoice with manuscript; pt watermark (18)32. Sheet: 125 x 200mmm (5 x 8"). Folds and creasing. £75 An invoice for candles and sooap from chandlers W. & J. Barton based in Bishopsgate. Stock: 43660 5. [Horses] R. Thwaiites, Ripon. To the 1. Design Adopted by the Council for the following places on Pleasure with a Pair of Univerity of London. Horses and Waiting [...] William Wilkins, M.A. R.A. Arch.t 1826. Printed by C. Smith & Greaves sc Birmingham [c.1820] Hullmandel. Engraving, sheet 65 x 100mm (2½ x 4"). Trimmed. Lithograph. Sheet 260 x 320mm (10¼ x 12½"). mss ''D' £60 added top right, some soiling. £190 Billhead for a man based in Ripon, Yorkshire, with The designs by William Wilkins RA (1778-1839) for horses for hire. With unicorn vignette and list of the main building of what is now University College distances to nearby towns. -

Télécharger Notre Brochure Voyages Individuels
Voyages Individuels 100 destinations sur 4 continents Asie | Océanie | Afrique | Moyen-Orient | Amérique Latine 2019-2020 Un Monde de Sensations Sensations vous présente sa brochure entièrement dédiée aux 103 destinations que nous vous proposons de découvrir en voyage individuel, de l’Asie à l’Australie, du Pacifique à l’Amérique Latine de l’Afrique au Moyen-Orient ! Sommaire 2 Une équipe, 15 ans de passion! 4 Voyager en Individuel 3 Sensations, c’est aussi... 5 ASIE OCEANIE MOYEN-ORIENT Arménie 8 Australie 160-163 Egypte 138-143 Bhoutan 24 Nouvelle-Zélande 164 Iran 156 Birmanie 54 Polynésie française 166 Israël 146 Cambodge 60-63 Jordanie 148 Chine 26 AFRIQUE Liban 150 Corée du Sud 36 Afrique du Sud 92-97 Maroc 144 Géorgie 10 Botswana 84 Oman 152-155 Indonésie 44-49 Cap Vert 104-107 Inde 18-21 Ethiopie 66 AMERIQUE LATINE Japon 30-35 Kenya 68-71 Argentine 132 Laos 56 Madagascar 98-101 Bolivie 126 Malaisie et Hong Kong 38 Namibie 86 Brésil 128-131 Malaisie Bornéo 40 Ouganda 78-81 Chili 134 Mongolie 28 Rwanda 82 Colombie 116 Népal 22 Sénégal 102 Costa Rica 112-115 Ouzbékistan 12 Tanzanie 72-77 Equateur 118-121 Philippines 42 Zimbabwe 88-91 Mexique 110 Sri Lanka 14-17 Pérou 122-125 Thaïlande 50-53 Vietnam 58-61 2 www.travel-sensations.com Avec son approche créative, Sensations s’est imposé rapidement comme le spécialiste du voyage sur mesure. Notre équipe se tient à votre disposition pour élaborer et réaliser de bout en bout le voyage qui vous correspond. Parce que l’on voyage tous différemment, votre projet mérite d’être personnalisé comme il se doit. -

Widespread Crater-Related Pitted Materials on Mars: Further Evidence for the Role of Target Volatiles During the Impact Process ⇑ Livio L
Icarus 220 (2012) 348–368 Contents lists available at SciVerse ScienceDirect Icarus journal homepage: www.elsevier.com/locate/icarus Widespread crater-related pitted materials on Mars: Further evidence for the role of target volatiles during the impact process ⇑ Livio L. Tornabene a, , Gordon R. Osinski a, Alfred S. McEwen b, Joseph M. Boyce c, Veronica J. Bray b, Christy M. Caudill b, John A. Grant d, Christopher W. Hamilton e, Sarah Mattson b, Peter J. Mouginis-Mark c a University of Western Ontario, Centre for Planetary Science and Exploration, Earth Sciences, London, ON, Canada N6A 5B7 b University of Arizona, Lunar and Planetary Lab, Tucson, AZ 85721-0092, USA c University of Hawai’i, Hawai’i Institute of Geophysics and Planetology, Ma¯noa, HI 96822, USA d Smithsonian Institution, Center for Earth and Planetary Studies, Washington, DC 20013-7012, USA e NASA Goddard Space Flight Center, Greenbelt, MD 20771, USA article info abstract Article history: Recently acquired high-resolution images of martian impact craters provide further evidence for the Received 28 August 2011 interaction between subsurface volatiles and the impact cratering process. A densely pitted crater-related Revised 29 April 2012 unit has been identified in images of 204 craters from the Mars Reconnaissance Orbiter. This sample of Accepted 9 May 2012 craters are nearly equally distributed between the two hemispheres, spanning from 53°Sto62°N latitude. Available online 24 May 2012 They range in diameter from 1 to 150 km, and are found at elevations between À5.5 to +5.2 km relative to the martian datum. The pits are polygonal to quasi-circular depressions that often occur in dense clus- Keywords: ters and range in size from 10 m to as large as 3 km. -

The Pancam Instrument for the Exomars Rover
ASTROBIOLOGY ExoMars Rover Mission Volume 17, Numbers 6 and 7, 2017 Mary Ann Liebert, Inc. DOI: 10.1089/ast.2016.1548 The PanCam Instrument for the ExoMars Rover A.J. Coates,1,2 R. Jaumann,3 A.D. Griffiths,1,2 C.E. Leff,1,2 N. Schmitz,3 J.-L. Josset,4 G. Paar,5 M. Gunn,6 E. Hauber,3 C.R. Cousins,7 R.E. Cross,6 P. Grindrod,2,8 J.C. Bridges,9 M. Balme,10 S. Gupta,11 I.A. Crawford,2,8 P. Irwin,12 R. Stabbins,1,2 D. Tirsch,3 J.L. Vago,13 T. Theodorou,1,2 M. Caballo-Perucha,5 G.R. Osinski,14 and the PanCam Team Abstract The scientific objectives of the ExoMars rover are designed to answer several key questions in the search for life on Mars. In particular, the unique subsurface drill will address some of these, such as the possible existence and stability of subsurface organics. PanCam will establish the surface geological and morphological context for the mission, working in collaboration with other context instruments. Here, we describe the PanCam scientific objectives in geology, atmospheric science, and 3-D vision. We discuss the design of PanCam, which includes a stereo pair of Wide Angle Cameras (WACs), each of which has an 11-position filter wheel and a High Resolution Camera (HRC) for high-resolution investigations of rock texture at a distance. The cameras and electronics are housed in an optical bench that provides the mechanical interface to the rover mast and a planetary protection barrier. -

March 21–25, 2016
FORTY-SEVENTH LUNAR AND PLANETARY SCIENCE CONFERENCE PROGRAM OF TECHNICAL SESSIONS MARCH 21–25, 2016 The Woodlands Waterway Marriott Hotel and Convention Center The Woodlands, Texas INSTITUTIONAL SUPPORT Universities Space Research Association Lunar and Planetary Institute National Aeronautics and Space Administration CONFERENCE CO-CHAIRS Stephen Mackwell, Lunar and Planetary Institute Eileen Stansbery, NASA Johnson Space Center PROGRAM COMMITTEE CHAIRS David Draper, NASA Johnson Space Center Walter Kiefer, Lunar and Planetary Institute PROGRAM COMMITTEE P. Doug Archer, NASA Johnson Space Center Nicolas LeCorvec, Lunar and Planetary Institute Katherine Bermingham, University of Maryland Yo Matsubara, Smithsonian Institute Janice Bishop, SETI and NASA Ames Research Center Francis McCubbin, NASA Johnson Space Center Jeremy Boyce, University of California, Los Angeles Andrew Needham, Carnegie Institution of Washington Lisa Danielson, NASA Johnson Space Center Lan-Anh Nguyen, NASA Johnson Space Center Deepak Dhingra, University of Idaho Paul Niles, NASA Johnson Space Center Stephen Elardo, Carnegie Institution of Washington Dorothy Oehler, NASA Johnson Space Center Marc Fries, NASA Johnson Space Center D. Alex Patthoff, Jet Propulsion Laboratory Cyrena Goodrich, Lunar and Planetary Institute Elizabeth Rampe, Aerodyne Industries, Jacobs JETS at John Gruener, NASA Johnson Space Center NASA Johnson Space Center Justin Hagerty, U.S. Geological Survey Carol Raymond, Jet Propulsion Laboratory Lindsay Hays, Jet Propulsion Laboratory Paul Schenk, -

Individuele Reizen 100 Bestemmingen Over 5 Continenten Afrika | Azië | Latijns-Amerika | Midden-Oosten | Oceanië 2019-2020 De Wereld Van Sensations
Travel Designer Individuele Reizen 100 bestemmingen over 5 continenten Afrika | Azië | Latijns-Amerika | Midden-Oosten | Oceanië 2019-2020 De Wereld van Sensations Een brochure gewijd aan 103 bestemmingen om weg te dromen van uw volgende reis. Individuele reizen of in kleine groep naar Azië, Afrika, Latijns-Amerika, het Midden-Oosten, Oceanië en de Pacific. Inhoud 2 Een team, 15 jaren vol passie ! 4 Individueel reizen 3 Sensations is ook… 5 AZIË AFRIKA MIDDEN-OOSTEN Armenië 8 Botswana 84 Egypte 138-143 Bhutan 24 Ethiopië 66 Iran 156 Cambodja 60-63 Kaapverdië 104-107 Israël 146 China 26 Kenia 68-71 Jordanië 148 Filipijnen 42 Madagaskar 98-101 Libanon 150 Georgië 10 Namibië 86 Marokko 144 India 18-21 Oeganda 78-81 Oman 152-155 Indonesië 44-49 Rwanda 82 Japan 30-35 Senegal 102 OCEANIË Laos 56 Tanzania 72-77 Australië 160-163 Maleisië en Hong Kong 38 Zimbabwe 88-91 Frans-Polynesië 166 Maleisisch Borneo 40 Zuid-Afrika 92-97 Nieuw-Zeeland 164 Mongolië 28 Myanmar 54 LATIJNS-AMERIKA Nepal 22 Argentinië 132 Oezbekistan 12 Bolivia 126 Sri Lanka 14-17 Brazilië 128-131 Thailand 50-53 Chili 134 Vietnam 58-61 Colombia 116 Zuid-Korea 36 Costa-Rica 112-115 Ecuador 118-121 Mexico 110 Peru 122-125 2 www.travel-sensations.com Ons team van Travel Designers staat tot uw beschikking om uw droomreis uit te werken en te realiseren. We werken een programma uit dat beantwoordt aan uw specifieke wensen en voorkeuren, rekening houdend met uw budget. U kiest voor een reis op maat of voor een van onze Kant-en-Klaar programma’s. -

Visible and Thermophysical Characteristics of the Best-Preserved Martian Craters, Part 2: Thermophysical Mapping of Resen and Noord
47th Lunar and Planetary Science Conference (2016) 2903.pdf VISIBLE AND THERMOPHYSICAL CHARACTERISTICS OF THE BEST-PRESERVED MARTIAN CRATERS, PART 2: THERMOPHYSICAL MAPPING OF RESEN AND NOORD. J. L. Piatek1, L. L. Torn- abene2, N. G. Barlow3, G. R. Osinski2,4, and S. J. Robbins5, 1Dept. of Geological Sciences, Central Connecticut State Univ., New Britain, CT ([email protected]) 2Centre for Planetary Science & Exploration (CPSX) and Dept. of Earth Sciences, Western University, London, ON, 3Dept. Physics and Astronomy, Northern Arizona Univ., Flagstaff, AZ, 4Dept. of Physics & Astronomy, Western University, London, ON, 5Southwest Research Institute, Boulder, CO. Introduction: This work is part of an ongoing continuous ejecta is not distinct in thermal images, it study to identify the characteristics associated with the may be classified into multiple units during our initial best-preserved craters on Mars. A list of the candidate mapping. Discontinuous ejecta units are defined based craters for this study were identified using criteria dis- on the appearance of thermophysically contrasted ma- cussed by [1]. Craters were prioritized by size; those of terial consistent with either ballistic emplacement of around the upper portion of simple-to-complex transi- ejecta (typically lower TI material forming secondary tion diameter (7-10 km) were selected for initial study, crater rays radial to the primary), and target material as smaller craters have less detail in lower resolution affected by airblast (typically higher TI). The inner data, while larger craters would introduce additional boundary of the discontinuous units is assumed to be complications such as variations in underlying target the continuous ejecta, while the outer boundary is the materials. -

Download Full-Text
Monday, October 31, 2005 Acute Lung Injury and ARDS SLIDE PRESENTATIONS 10:30 AM - 12:00 PM ALVEOLAR FLUID REABSORPTION IS IMPAIRED BY HYPER- CONCLUSION: Large intraoperative Vt and larger volumes of intra- CAPNIA INDEPENDENTLY OF EXTRACELLULAR AND IN- venous fluids during pneumonectomy are associated with an increased TRACELLULAR PH risk of post-operative respiratory failure. Arturo Briva MD* Lynn Welch BS Jiwang Chen PhD Pavlos Myrianthefs CLINICAL IMPLICATIONS: Potentially harmful intraoperative MD Zaher Azzam MD Emilia Lecuona PhD Vidas Dumasius BS Daniel ventilatory settings, in particular large tidal volumes, should be avoided Batlle MD Yosef Gruenbaum PhD Jacob I. Sznajder MD Northwestern during pneumonectomy. University, Chicago, IL DISCLOSURE: Evans Ferna´ndez, None. PURPOSE: Alveolar epithelium is exposed to high CO2 tensions (hypercapnia) in patients with COPD and during permissive hypercapnia in mechanically ventilated subjects. Recently, some reports propose that hypercapnia could be beneficial in the treatment of ALI/ARDS. However, more recently new data has been presented suggesting that hypercapnia may have deleterious effects on the pulmonary epithelium. The objective of our investigation was to determine the effects of hypercapnia on alveolar epithelial function. METHODS: Alveolar fluid reabsorption (AFR) was assessed during hypercapnia with normal and acid pH as compared to metabolic acidosis in the isolated rat lung model. In parallel, Na,K-ATPase activity and protein abundance in alveolar type II cell cultures was evaluated. RESULTS: Hypercapnia decreased AFR by ϳ 60% (pCO2 ϳ 80 mmHg, pH ϭ 7.15). With high pCO2 even at normal pH (7.40) alveolar fluid reabsorption was decreased but not during metabolic acidosis (pH 7.2 and normal PCO2). -

Namen Van Sterrenbeelden En Sterren
Prof. Dr. Ρ. Η. van Laer VREEMDE WOORDEN IN DE STERRENKUNDE en namen van sterrenbeelden en sterren Tweede herziene druk J. B. Wolters Groningen 1964 Uitgegeven met steun van het Prins Bernhardfonds Inhoud Blz. Voorrede van Prof. Minnaert 5 Verantwoording 7 Lijst van de gebruikte afkortingen en tekens 11 Het Griekse alfabet 12 Transcriptie en uitspraak van Arabische woorden . 13 Uitspraak van de Griekse en Latijnse woorden 14 EERSTE AFDELING Vreemde woorden in de sterrenkunde 19 TWEEDE AFDELING Namen van sterrenbeelden en sterren 71 Historische inleiding 71 Verklaring van de namen van sterrenbeelden en sterren ... 78 Planeten en hun manen 107 Planetoïden 112 De maan 114 Kometen 117 Meteoorzwermen 118 INDICES Nederlandse namen van sterrenbeelden 119 Lijst van de behandelde namen van sterren, planeten, planetoïden en manen 122 3 Voorrede van Prof \ Dr. M. G. /. Minnaert bij de eerste druk Het gebruik van vreemde woorden in de wetenschap heeft zijn kwade, maar ook zijn goede zijde. Het is een euvel, daar het de begrijpelijkheid van de tekst voor den niet-geschoolden lezer bemoeilijkt, de stijl onper- soonlijker en vlakker maakt. Anderzijds is het een voordeel, daar de vast- stelling van internationaal gangbare, nauwkeurig gedefinieerde termen de scherpe vastlegging der begrippen bevordert, en de betrekkingen verge- makkelijkt tussen de wetenschappelijke werkers over de gehele wereld. Het werk van Dr. van Laer nu is bij uitstek geschikt om tegemoet te ko- men aan de bezwaren, en de voordelen tot hun recht te laten komen. Hij brengt ons vooreerst een korte, heldere verklaring van wat elk vreemd woord in onze wetenschap betekent: een belangrijk hulpmiddel dus voor ieder, die zich in de Sterrekunde inwerken wil. -
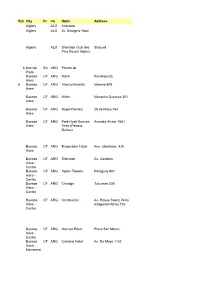
Rat E City Pr Co Hotel Address Algiers ALG Sheraton Algiers ALG St
Rat City Pr Co Hotel Address e Algiers ALG Sheraton Algiers ALG St. George's Hotel Algiers ALG Sheraton Club des Staoueli Pins Resort Algiers 6 Mar del BA ARG Provincial Plata Buenos CF ARG Meliá Reconquista Aires 6 Buenos CF ARG Intercontinental Moreno 809 Aires Buenos CF ARG Hilton Macacha Guemes 351 Aires Buenos CF ARG Regal Pacifico 25 de Mayo 764 Aires Buenos CF ARG Park Hyatt Buenos Avenida Alvear 1661 Aires Aires (Palacio Duhau) Buenos CF ARG Emperador Hotel Ave. Libertador, 420 Aires Buenos CF ARG Sheraton Av. Cordoba Aires - Centro Buenos CF ARG Aspen Towers Paraguay 857 Aires - Centro Buenos CF ARG Claridge Tucuman 535 Aires - Centro Buenos CF ARG Continental Av. Roque Saenz Peña Aires - (Diagonal Norte) 725 Centro Buenos CF ARG Marriott Plaza Plaza San Mertin Aires - Centro Buenos CF ARG Castelar Hotel Av. De Mayo 1152 Aires - Monserrat 5 Buenos CF ARG Alan Faena Martha Salotti 445 Aires - Puerto Madero 7 Buenos CF ARG Recoleta Calle J. L. Pagano 2684 Aires - REcoleta Buenos CF ARG Four Seasons Recova Aires - Recoleta Buenos CF ARG De Alvear Marcel T. de Alvear Aires - Recoleta Buenos CF ARG Sheraton Buenos Plaza de los Ingleses Aires - Aires Retiro 6 Mendoza CU ARG Aconcagua San Lorenzo 545 (cerca de Plaza de Italia) Mendoza CU ARG Sheraton Mendoza CU ARG Premium Tower Puerto MI ARG Sheraton Iguazu Puerto MI ARG Panoramic Paraguay 372 Iguazu Puerto MI ARG Iguazu Grand Hotel Iguazu Puerto MI ARG Esturion Iguazu Puerto MI ARG Saint George Iguazu Cachi SA ARG Sala de Payogasta Ruta Nac. -

Verslag Vendelinusvergadering 14-01-2017 Heel Wat Volk Was Komen Opdagen. Daniël Verjaarde: Een Dikke Proficiat Gewenst! De
Verslag Vendelinusvergadering 14-01-2017 Heel wat volk was komen opdagen. Daniël verjaarde: een dikke proficiat gewenst! De traktatie smaakte heerlijk. De fullerenen die hier zijn afgebeeld doen aan kerstballen denken en het leek mij leuk bij ons kerstfeestje ze even ter sprake te brengen. Bedenk wel, ze zijn onzichtbaar klein, maar de afbeelding suggereert wonderen en leek me daardoor geschikt voor het wondere kerstfeest. Het lukte mij net niet tijdens het kerstfeestje over de nanoballetjes te klappen daarom op 14 januari het mini praatje. Theoretisch moesten er zoiets bestaan als holle koolstof moleculen, daar werd al tientallen jaren over gedacht. De synthese werd pas in 1985 door Harry Kroto, Robert Curl en Richard Smalley in het laboratorium tot stand gebracht. ( Nobel Prijs in 1996!!). Het denken over deze polycyclische koolstofmoleculen ging lang vooraf aan de constructie. Grafiet kun je je voorstellen als dunne laagjes grafeen. Grafeen bestaat in die voorstelling uit een laagje van verbonden zeshoekige en vijfhoekige ringen. Op die hoekpunten zit telkens een koolstofatoom. De oorspronkelijk zeshoekige ringen kunnen door straling vijfhoekig worden. Maar vijfhoekige vormen blijven niet naast zeshoekige in een plat vlak liggen, als je het vlak wilt opvullen, passen de vijfhoekige niet en de combinatie vijf zes veroorzaakt kromming, voetballen of fullerenen. Fullerenen zijn holle gesloten moleculen van koolstofatomen, die in vijf of zes hoekige ringen opgebouwd zijn. Ze werden al lang theoretisch verondersteld. Wat hier zo in de gauwigheid gezegd wordt over buckeyballs is een wetenschappelijk proces van vele decennia geweest De oude bekende verschijningsvormen in de natuur van het element koolstof (allotropen is het officiële woord) zijn bijvoorbeeld amorf koolstof : roet, verder diamant, grafiet, ( dat weer opgebouwd is uit dunne laagjes), grafeen. -

1 Synthetic Topography from the Decameter to the Centimeter Scale on Mars for Scientific 2 and Rover Operations of the ESA-Roscosmos Exomars Mission 3 4 O
1 Synthetic topography from the decameter to the centimeter scale on Mars for scientific 2 and rover operations of the ESA-Roscosmos ExoMars mission 3 4 O. Ruesch1,2, M. Hess3, K. Wohlfarth3, T. Heyer1, C. Wöhler3, T. R. R. Bontognali4,5, C. Orgel2, 5 E. Sefton-Nash2, J.-L. Josset4, J. L. Vago2 6 7 1Institut für Planetologie, Westfälische-Wilhelms Universität, Münster, Germany. 8 2European Space and Technology Center (ESTEC), European Space Agency (ESA), Noord- 9 wijk, The Netherlands. 10 3Image Analysis Group, Technische Universität Dortmund, Dortmund, Germany. 11 4Space Exploration Institute, Fbg de l’Hopital 68, 2002 Neuchâtel, Switzerland 12 5Dep. of Environmental Sciences, Univ. of Basel, Klingelbergstr. 27, 4056 Basel, Switzer- 13 land 14 15 Corresponding author: 16 Dr. Ottaviano Ruesch 17 Institut für Planetologie 18 Westfälische Wilhelms Universität Münster 19 Wilhelm-Klemm-Straße 10 20 48149 Münster 21 Deutschland 22 Email: [email protected] 23 24 Keywords 25 • Topographic modelling 26 • Martian surface 27 • Image simulation 28 29 30 31 32 33 1 34 0. Abstract 35 The ESA-Roscosmos ExoMars platform and rover mission will have complex interactions with 36 the martian surface. In order to plan and perform engineering and scientific operations, the 37 morphological characteristics of the terrain surrounding the rover need to be characterized from 38 the decameter to the centimeter spatial scale. The smallest possible features currently identifi- 39 able are typically >0.75 m in size, corresponding to three times the ground sampling resolution 40 of the High Resolution Imaging Science Experiment (HiRISE) camera on NASA’s Mars Re- 41 connaissance Orbiter (MRO).