Activation of GPR56, a Novel Adhesion GPCR, Is Necessary for Nuclear Androgen Receptor Signaling in Prostate Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Killer-Like Receptors and GPR56 Progressive Expression Defines Cytokine Production of Human CD4+ Memory T Cells
ARTICLE https://doi.org/10.1038/s41467-019-10018-1 OPEN Killer-like receptors and GPR56 progressive expression defines cytokine production of human CD4+ memory T cells Kim-Long Truong1,7, Stephan Schlickeiser1,2,7, Katrin Vogt1, David Boës1, Katarina Stanko1, Christine Appelt1, Mathias Streitz1, Gerald Grütz1,2, Nadja Stobutzki1, Christian Meisel1, Christina Iwert1, Stefan Tomiuk3, Julia K. Polansky2,4, Andreas Pascher5, Nina Babel2,6, Ulrik Stervbo 6, Igor Sauer 5, Undine Gerlach5 & Birgit Sawitzki1,2 1234567890():,; All memory T cells mount an accelerated response on antigen reencounter, but significant functional heterogeneity is present within the respective memory T-cell subsets as defined by CCR7 and CD45RA expression, thereby warranting further stratification. Here we show that several surface markers, including KLRB1, KLRG1, GPR56, and KLRF1, help define low, high, or exhausted cytokine producers within human peripheral and intrahepatic CD4+ memory T-cell populations. Highest simultaneous production of TNF and IFN-γ is observed in KLRB1+KLRG1+GPR56+ CD4 T cells. By contrast, KLRF1 expression is associated with T-cell exhaustion and reduced TNF/IFN-γ production. Lastly, TCRβ repertoire analysis and in vitro differentiation support a regulated, progressive expression for these markers during CD4+ memory T-cell differentiation. Our results thus help refine the classification of human memory T cells to provide insights on inflammatory disease progression and immunotherapy development. 1 Institute of Medical Immunology, Charité – Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin and Berlin Institute of Health, 13353 Berlin, Germany. 2 Berlin-Brandenburg Center for Regenerative Therapies (BCRT), Charité – Universitätsmedizin Berlin, 13353 Berlin, Germany. 3 Milteny Biotec GmbH, 51429 Bergisch Gladbach, Germany. -

The Orphan Receptor GPR17 Is Unresponsive to Uracil Nucleotides and Cysteinyl Leukotrienes S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/02/mol.116.107904.DC1 1521-0111/91/5/518–532$25.00 https://doi.org/10.1124/mol.116.107904 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:518–532, May 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics The Orphan Receptor GPR17 Is Unresponsive to Uracil Nucleotides and Cysteinyl Leukotrienes s Katharina Simon, Nicole Merten, Ralf Schröder, Stephanie Hennen, Philip Preis, Nina-Katharina Schmitt, Lucas Peters, Ramona Schrage,1 Celine Vermeiren, Michel Gillard, Klaus Mohr, Jesus Gomeza, and Evi Kostenis Molecular, Cellular and Pharmacobiology Section, Institute of Pharmaceutical Biology (K.S., N.M., Ral.S., S.H., P.P., N.-K.S, L.P., J.G., E.K.), Research Training Group 1873 (K.S., E.K.), Pharmacology and Toxicology Section, Institute of Pharmacy (Ram.S., K.M.), University of Bonn, Bonn, Germany; UCB Pharma, CNS Research, Braine l’Alleud, Belgium (C.V., M.G.). Downloaded from Received December 16, 2016; accepted March 1, 2017 ABSTRACT Pairing orphan G protein–coupled receptors (GPCRs) with their using eight distinct functional assay platforms based on label- cognate endogenous ligands is expected to have a major im- free pathway-unbiased biosensor technologies, as well as molpharm.aspetjournals.org pact on our understanding of GPCR biology. It follows that the canonical second-messenger or biochemical assays. Appraisal reproducibility of orphan receptor ligand pairs should be of of GPR17 activity can be accomplished with neither the coapplica- fundamental importance to guide meaningful investigations into tion of both ligand classes nor the exogenous transfection of partner the pharmacology and function of individual receptors. -

Edinburgh Research Explorer
Edinburgh Research Explorer International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list Citation for published version: Davenport, AP, Alexander, SPH, Sharman, JL, Pawson, AJ, Benson, HE, Monaghan, AE, Liew, WC, Mpamhanga, CP, Bonner, TI, Neubig, RR, Pin, JP, Spedding, M & Harmar, AJ 2013, 'International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands', Pharmacological reviews, vol. 65, no. 3, pp. 967-86. https://doi.org/10.1124/pr.112.007179 Digital Object Identifier (DOI): 10.1124/pr.112.007179 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Pharmacological reviews Publisher Rights Statement: U.S. Government work not protected by U.S. copyright General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 02. Oct. 2021 1521-0081/65/3/967–986$25.00 http://dx.doi.org/10.1124/pr.112.007179 PHARMACOLOGICAL REVIEWS Pharmacol Rev 65:967–986, July 2013 U.S. -

Protein Identities in Evs Isolated from U87-MG GBM Cells As Determined by NG LC-MS/MS
Protein identities in EVs isolated from U87-MG GBM cells as determined by NG LC-MS/MS. No. Accession Description Σ Coverage Σ# Proteins Σ# Unique Peptides Σ# Peptides Σ# PSMs # AAs MW [kDa] calc. pI 1 A8MS94 Putative golgin subfamily A member 2-like protein 5 OS=Homo sapiens PE=5 SV=2 - [GG2L5_HUMAN] 100 1 1 7 88 110 12,03704523 5,681152344 2 P60660 Myosin light polypeptide 6 OS=Homo sapiens GN=MYL6 PE=1 SV=2 - [MYL6_HUMAN] 100 3 5 17 173 151 16,91913397 4,652832031 3 Q6ZYL4 General transcription factor IIH subunit 5 OS=Homo sapiens GN=GTF2H5 PE=1 SV=1 - [TF2H5_HUMAN] 98,59 1 1 4 13 71 8,048185945 4,652832031 4 P60709 Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 - [ACTB_HUMAN] 97,6 5 5 35 917 375 41,70973209 5,478027344 5 P13489 Ribonuclease inhibitor OS=Homo sapiens GN=RNH1 PE=1 SV=2 - [RINI_HUMAN] 96,75 1 12 37 173 461 49,94108966 4,817871094 6 P09382 Galectin-1 OS=Homo sapiens GN=LGALS1 PE=1 SV=2 - [LEG1_HUMAN] 96,3 1 7 14 283 135 14,70620005 5,503417969 7 P60174 Triosephosphate isomerase OS=Homo sapiens GN=TPI1 PE=1 SV=3 - [TPIS_HUMAN] 95,1 3 16 25 375 286 30,77169764 5,922363281 8 P04406 Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 - [G3P_HUMAN] 94,63 2 13 31 509 335 36,03039959 8,455566406 9 Q15185 Prostaglandin E synthase 3 OS=Homo sapiens GN=PTGES3 PE=1 SV=1 - [TEBP_HUMAN] 93,13 1 5 12 74 160 18,68541938 4,538574219 10 P09417 Dihydropteridine reductase OS=Homo sapiens GN=QDPR PE=1 SV=2 - [DHPR_HUMAN] 93,03 1 1 17 69 244 25,77302971 7,371582031 11 P01911 HLA class II histocompatibility antigen, -
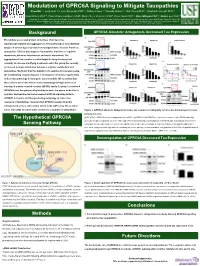
Background the Hypothetical GPRC6A Sensing Pathway
Modulation of GPRC6A Signaling to Mitigate Tauopathies Chao Ma1,2, Jerry Hunt1,3, Leslie Sandusky PhD1,3, William Fraser1,3, Ronald Alvarez1,3, Dali Zheng PhD1,4, Siddharth Kamath PhD1,2, Chad Dickey PhD1,4, Hans Bräuner-Osborne PhD5, Daniel Sejer Pedersen PhD5, Kevin Nash PhD1,2, Dave Morgan PhD1,2, Daniel Lee PhD1,3 1:Byrd Alzheimer‘s Institute, University of South Florida, Tampa, FL 33613, USA. 2:Morsani College of Medicine, Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, FL 33612, USA. 3:College of Pharmacy, Department of Pharmaceutical Sciences, University of South Florida, Tampa, FL 33612, USA. 4:Morsani College of Medicine, Department of Molecular Medicine, University of South Florida, Tampa, FL 33612, USA. 5:Department of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Jagtvej 162, 2100 Copenhagen, Denmark Background GPRC6A Allosteric Antagonists Decreased Tau Expression Microtubule associated protein named tau, often becomes hyperphosphorylated and aggregates to form pathological neurofibrillary tangles in several age-associated neurodegenerative diseases known as tauopathies. Clinical phenotypes of tauopathies manifest as cognitive impairment, behavior disturbances and motor impairment. The aggregation of tau remains a central target for drug discovery, but currently no disease-modifying treatments exist. Our group has recently uncovered a unique interaction between L-arginine metabolism and tauopathies. We found that the depletion of L-arginine by overexpressing the metabolizing enzyme arginase 1 and arginine deiminase significantly reduced tau pathology in transgenic mouse models. We speculate that these effects associate with increased autophagy through amino acid sensing. G protein-coupled receptor (GPCR), family C, group 6, member A (GPRC6A) was deorphanized by binding to basic L-α amino acids, like L- arginine. -

Progastrin Production Transitions from Bmi1+/Prox1+ to Lgr5high Cells During Early Intestinal Tumorigenesis Julie Giraud, M
Progastrin production transitions from Bmi1+/Prox1+ to Lgr5high cells during early intestinal tumorigenesis Julie Giraud, M. Foroutan, Jihane Boubaker-Vitre, Fanny Grillet, Z. Homayed, U. Jadhav, Philippe Crespy, Cyril Breuker, J-F. Bourgaux, J. Hazerbroucq, et al. To cite this version: Julie Giraud, M. Foroutan, Jihane Boubaker-Vitre, Fanny Grillet, Z. Homayed, et al.. Progastrin production transitions from Bmi1+/Prox1+ to Lgr5high cells during early intestinal tumorigene- sis. Translational Oncology, Elsevier, 2020, 14 (2), pp.101001. 10.1016/j.tranon.2020.101001. hal- 03090781 HAL Id: hal-03090781 https://hal.archives-ouvertes.fr/hal-03090781 Submitted on 12 Jan 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License Translational Oncology 14 (2021) 101001 Contents lists available at ScienceDirect Translational Oncology journal homepage: www.elsevier.com/locate/tranon Original Research + + high Progastrin production transitions from Bmi1 /Prox1 to Lgr5 cells during early intestinal tumorigenesis J. Giraud a, M. Foroutan b,c,1, J. Boubaker-Vitre a,1, F. Grillet a, Z. Homayed a, U. Jadhav d, P. Crespy a, C. Breuker a, J-F. -

Pancancer Progression Human Vjune2017
Gene Symbol Accession Alias/Prev Symbol Official Full Name AAMP NM_001087.3 - angio-associated, migratory cell protein ABI3BP NM_015429.3 NESHBP|TARSH ABI family, member 3 (NESH) binding protein ACHE NM_000665.3 ACEE|ARACHE|N-ACHE|YT acetylcholinesterase ACTG2 NM_001615.3 ACT|ACTA3|ACTE|ACTL3|ACTSG actin, gamma 2, smooth muscle, enteric ACVR1 NM_001105.2 ACTRI|ACVR1A|ACVRLK2|ALK2|FOP|SKR1|TSRI activin A receptor, type I ACVR1C NM_145259.2 ACVRLK7|ALK7 activin A receptor, type IC ACVRL1 NM_000020.1 ACVRLK1|ALK-1|ALK1|HHT|HHT2|ORW2|SKR3|TSR-I activin A receptor type II-like 1 ADAM15 NM_207195.1 MDC15 ADAM metallopeptidase domain 15 ADAM17 NM_003183.4 ADAM18|CD156B|CSVP|NISBD|TACE ADAM metallopeptidase domain 17 ADAM28 NM_014265.4 ADAM 28|ADAM23|MDC-L|MDC-Lm|MDC-Ls|MDCL|eMDC II|eMDCII ADAM metallopeptidase domain 28 ADAM8 NM_001109.4 CD156|MS2 ADAM metallopeptidase domain 8 ADAM9 NM_001005845.1 CORD9|MCMP|MDC9|Mltng ADAM metallopeptidase domain 9 ADAMTS1 NM_006988.3 C3-C5|METH1 ADAM metallopeptidase with thrombospondin type 1 motif, 1 ADAMTS12 NM_030955.2 PRO4389 ADAM metallopeptidase with thrombospondin type 1 motif, 12 ADAMTS8 NM_007037.4 ADAM-TS8|METH2 ADAM metallopeptidase with thrombospondin type 1 motif, 8 ADAP1 NM_006869.2 CENTA1|GCS1L|p42IP4 ArfGAP with dual PH domains 1 ADD1 NM_001119.4 ADDA adducin 1 (alpha) ADM2 NM_001253845.1 AM2|dJ579N16.4 adrenomedullin 2 ADRA2B NM_000682.4 ADRA2L1|ADRA2RL1|ADRARL1|ALPHA2BAR|alpha-2BAR adrenoceptor alpha 2B AEBP1 NM_001129.3 ACLP AE binding protein 1 AGGF1 NM_018046.3 GPATC7|GPATCH7|HSU84971|HUS84971|VG5Q -

GPR56 Regulates VEGF Production and Angiogenesis During Melanoma Progression
Published OnlineFirst July 1, 2011; DOI: 10.1158/0008-5472.CAN-10-4543 Cancer Tumor and Stem Cell Biology Research GPR56 Regulates VEGF Production and Angiogenesis during Melanoma Progression Liquan Yang1, Guangchun Chen1, Sonali Mohanty1, Glynis Scott2, Fabeha Fazal3, Arshad Rahman3, Shahinoor Begum4, Richard O. Hynes4, and Lei Xu1,2 Abstract Angiogenesis is a critical step during cancer progression. The VEGF is a major stimulator for angiogenesis and is predominantly contributed by cancer cells in tumors. Inhibition of the VEGF signaling pathway has shown promising therapeutic benefits for cancer patients, but adaptive tumor responses are often observed, indicating the need for further understanding of VEGF regulation. We report that a novel G protein–coupled receptor, GPR56, inhibits VEGF production from the melanoma cell lines and impedes melanoma angiogen- esis and growth, through the serine threonine proline-rich segment in its N-terminus and a signaling pathway involving protein kinase Ca. We also present evidence that the two fragments of GPR56, which are generated by autocatalyzed cleavage, played distinct roles in regulating VEGF production and melanoma progression. Finally, consistent with its suppressive roles in melanoma progression, the expression levels of GPR56 are inversely correlated with the malignancy of melanomas in human subjects. We propose that components of the GPR56-mediated signaling pathway may serve as new targets for antiangiogenic treatment of melanoma. Cancer Res; 71(16); 1–11. Ó2011 AACR. Introduction its occurrence strongly argues for combinations of antiangio- genic regimens to effectively treat cancer. Angiogenesis is a process of nascent blood vessel formation VEGF is a potent growth factor for angiogenesis and is a (1) and is critical for tumor growth and metastasis (2). -

The N-Terminus of the Saccharomyces Cerevisiae G Protein-Coupled Receptor Ste2p: Formation of Dimer Interfaces and Negative Regulation
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2013 The N-terminus of the Saccharomyces cerevisiae G protein- coupled receptor Ste2p: formation of dimer interfaces and negative regulation Mohammad Seraj Uddin [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Biochemistry Commons, Molecular Biology Commons, and the Structural Biology Commons Recommended Citation Uddin, Mohammad Seraj, "The N-terminus of the Saccharomyces cerevisiae G protein-coupled receptor Ste2p: formation of dimer interfaces and negative regulation. " PhD diss., University of Tennessee, 2013. https://trace.tennessee.edu/utk_graddiss/2490 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Mohammad Seraj Uddin entitled "The N- terminus of the Saccharomyces cerevisiae G protein-coupled receptor Ste2p: formation of dimer interfaces and negative regulation." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Microbiology. Jeffrey -

An Overview on G Protein-Coupled Receptor-Induced Signal Transduction in Acute Myeloid Leukemia
An overview on G protein-coupled receptor-induced signal transduction in Acute Myeloid Leukemia 1* 1,3 4,5,6 2* Frode Selheim , Elise Aasebø , Catalina Ribas and Anna M. Aragay 1The Proteomics Unit at the University of Bergen, Department of Biomedicine, University of Bergen, Jonas Lies vei 91, 5020 Bergen, Norway; 3 Department of Clinical Science, University of Bergen, Jonas Lies vei 87, 5021 Bergen, Norway; [email protected]. 2Departamento de Biologia Celular. Instituto de Biología Molecular de Barcelona (IBMB-CSIC), Spanish National Research Council (CSIC), Baldiri i Reixac, 15, 08028 Barcelona, Spain; [email protected]. 4Departamento de Biología Molecular and Centro de Biología Molecular “Severo Ochoa” (UAM-CSIC), 28049 Madrid, Spain; 5Instituto de Investigación Sanitaria La Princesa, 28006 Madrid, Spain; 6CIBER de Enfermedades Cardiovasculares, ISCIII (CIBERCV), 28029 Madrid, Spain, [email protected] * Corresponding authors: Frode Selheim Adr: Jonas Lies vei 91, 5020 Bergen, Norway Email: [email protected], Tel:+4755586091 Anna M. Aragay Adr: Baldiri i Reixac, 15, 08028 Barcelona. Spain. E-mail: [email protected]; Tel.: +934098671 1 Abstract Background: Acute myeloid leukemia (AML) is a genetically heterogeneous disease characterized by uncontrolled proliferation of precursor myeloid-lineage cells in the bone marrow. AML is also characterized with patients with poor long-term survival outcomes due to relapse. Many efforts have been made to understand the biological heterogeneity of AML and the challenges to develop new therapies are therefore enormous. G protein-coupled receptors (GPCRs) are a large attractive drug targeted family of transmembrane proteins, and aberrant GPCR expression and GPCR-mediated signaling have been implicated in leukemogenesis of AML. -

The Adhesion G Protein-Coupled Receptor GPR56 Is a Cell-Autonomous Regulator of Oligodendrocyte Development
ARTICLE Received 27 May 2014 | Accepted 14 Dec 2014 | Published 21 Jan 2015 DOI: 10.1038/ncomms7121 OPEN The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development Stefanie Giera1,*, Yiyu Deng1,*,w, Rong Luo1,*, Sarah D. Ackerman2, Amit Mogha2, Kelly R. Monk2,3, Yanqin Ying1, Sung-Jin Jeong1,w, Manabu Makinodan4,5, Allison R. Bialas4,5, Bernard S. Chang6, Beth Stevens4,5, Gabriel Corfas4,5,w & Xianhua Piao1 Mutations in GPR56, a member of the adhesion G protein-coupled receptor family, cause a human brain malformation called bilateral frontoparietal polymicrogyria (BFPP). Magnetic resonance imaging (MRI) of BFPP brains reveals myelination defects in addition to brain malformation. However, the cellular role of GPR56 in oligodendrocyte development remains unknown. Here, we demonstrate that loss of Gpr56 leads to hypomyelination of the central nervous system in mice. GPR56 levels are abundant throughout early stages of oligodendrocyte development, but are downregulated in myelinating oligodendrocytes. Gpr56-knockout mice manifest with decreased oligodendrocyte precursor cell (OPC) proliferation and diminished levels of active RhoA, leading to fewer mature oligodendrocytes and a reduced number of myelinated axons in the corpus callosum and optic nerves. Conditional ablation of Gpr56 in OPCs leads to a reduced number of mature oligodendrocytes as seen in constitutive knockout of Gpr56. Together, our data define GPR56 as a cell-autonomous regulator of oligodendrocyte development. 1 Division of Newborn Medicine, Department of Medicine, Boston Children’s Hospital and Harvard Medical School, Boston, Massachusetts 02115, USA. 2 Department of Developmental Biology, Washington University School of Medicine, St Louis, Missouri 63110, USA. -

G Protein-Coupled Receptors
S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2015/16: G protein-coupled receptors. British Journal of Pharmacology (2015) 172, 5744–5869 THE CONCISE GUIDE TO PHARMACOLOGY 2015/16: G protein-coupled receptors Stephen PH Alexander1, Anthony P Davenport2, Eamonn Kelly3, Neil Marrion3, John A Peters4, Helen E Benson5, Elena Faccenda5, Adam J Pawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, NG7 2UH, UK, 2Clinical Pharmacology Unit, University of Cambridge, Cambridge, CB2 0QQ, UK, 3School of Physiology and Pharmacology, University of Bristol, Bristol, BS8 1TD, UK, 4Neuroscience Division, Medical Education Institute, Ninewells Hospital and Medical School, University of Dundee, Dundee, DD1 9SY, UK, 5Centre for Integrative Physiology, University of Edinburgh, Edinburgh, EH8 9XD, UK Abstract The Concise Guide to PHARMACOLOGY 2015/16 provides concise overviews of the key properties of over 1750 human drug targets with their pharmacology, plus links to an open access knowledgebase of drug targets and their ligands (www.guidetopharmacology.org), which provides more detailed views of target and ligand properties. The full contents can be found at http://onlinelibrary.wiley.com/doi/ 10.1111/bph.13348/full. G protein-coupled receptors are one of the eight major pharmacological targets into which the Guide is divided, with the others being: ligand-gated ion channels, voltage-gated ion channels, other ion channels, nuclear hormone receptors, catalytic receptors, enzymes and transporters. These are presented with nomenclature guidance and summary information on the best available pharmacological tools, alongside key references and suggestions for further reading.