Food and Drug Addictions: Similarities and Differences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Short Note on Dark Side of Addiction
lism and D OPEN ACCESS Freely available online o ru h g o lc D A e p f e o n l d a e Journal of n r n c u e o J ISSN: 2329-6488 Alcoholism & Drug Dependence Editorial Short Note on Dark Side of Addiction Richard Samson* Department of Psychology, Faculty of Pharmacy, University of Michigan, 500 S State St, Ann Arbor, MI 48109, United States ABSTRACT Addiction is a biopsychosocial disorder characterized by repeated use of drugs, or repetitive engagement in a behavior such as gambling, despite harm to self and others. According to the "brain disease model of addiction," while a number of psychosocial factors contribute to the development and maintenance of addiction, a biological process that is induced by repeated exposure to an addictive stimulus is the core pathology that drives the development and maintenance of an addiction. Many scholars who study addiction argue that the brain disease model is incomplete and misleading. Emotions are "feeling" states and classic physiological emotive responses that are interpreted based on the history of the organism and the context. Motivation is a persistent state that leads to organized activity. Both are intervening variables and intimately related and have neural representations in the brain. The present thesis is that drugs of abuse elicit powerful emotions that can be interwoven conceptually into this framework. Keywords: Allostasis; Corticotropin-releasing factor; Dynorphin; Extended amygdala; Incentive salience; Opponent process INTRODUCTION clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form The brain disease model posits that addiction is a disorder of the of sexual intercourse – as an addiction (i.e., sexual addiction). -

An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population
Medical University of South Carolina MEDICA MUSC Theses and Dissertations 2017 An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population Carley Garris Lovell Medical University of South Carolina Follow this and additional works at: https://medica-musc.researchcommons.org/theses Recommended Citation Lovell, Carley Garris, "An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population" (2017). MUSC Theses and Dissertations. 369. https://medica-musc.researchcommons.org/theses/369 This Dissertation is brought to you for free and open access by MEDICA. It has been accepted for inclusion in MUSC Theses and Dissertations by an authorized administrator of MEDICA. For more information, please contact [email protected]. ! ! An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population Carley Garris Lovell A dissertation submitted to the faculty of the Medical University of South Carolina in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Nursing. September 15, 2017 ii! ! ACKNOWLEDGEMENTS I would like to thank my dissertation committee: Drs. Gayenell Magwood, Amy Adkins, Martina Mueller, and Dace Svikis. Dr. Magwood has been my advisor during my entire course of study, and her consistent encouragement and support as my chair allowed me to successfully complete my studies. She supplied valuable guidance throughout the journey, literally starting from day one. I appreciate her invaluable support. Dr. Mueller is one of the clearest thinkers that I have met, and I value her contributions to my study immensely. -

Surprise-Flavors-120620.Pdf
BANANA CREAM PIE CHOCOHOLIC CHUNK COCONUT CREAM PIE banana ice cream with marshmallow ripple deep dark chocolate ice cream coconut ice cream with marshmallow ripple, and sugar cookie gems with dark chocolate chips coconut flakes and sugar cookie gems BIRTHDAY CAKE CHOCOLATE COFFEE CHOCOLATE CHIP cake batter ice cream chocolate coffee flavored ice cream with a blue butter cream ripple and sprinkles flavored ice cream with chocolate chips BLUE MONSTER CHOCOLATE CHIP COOKIE DOUGH COTTON CANDY blue vanilla ice cream with Oreo ® cookies vanilla ice cream with cookie dough pieces cotton candy flavored ice cream and Nestle ® chocolate chip cookies and chocolate chips with mini chocolate rainbow chips BLUEBERRY COBBLER CHOCOLATE MALT WITH CARAMEL DEEP DISH APPLE PIE vanilla ice cream malted chocolate ice cream apple flavored ice cream with apple sauce ripple with blueberry ripple and sugar cookie gems with caramel ripple and cinnamon crunch pieces BUCKEYE CHOCOLATE PEANUT BUTTER BROWNIE EGGNOG peanut butter ice cream chocolate ice cream with Reese’s Peanut Butter® ripple eggnog with fudge ripple and buckeye candy pieces and brownie chunks flavored ice cream BUTTER PECAN CHOCOLATE PECAN GRAHAM CENTRAL STATION butter pecan flavored ice cream chocolate ice cream graham flavored ice cream with a graham cracker ripple with whole roasted, buttered and salted pecans with whole, roasted, buttered and salted pecans and chocolate covered crunchies CARAMEL APPLE CINNAMON ROLL HEAVENLY HASH sour apple flavored ice cream cake batter flavored ice cream chocolate -
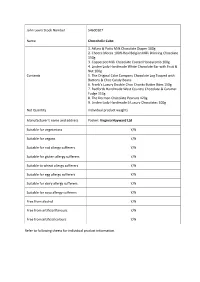
John Lewis Stock Number 54600107 Name Chocoholic Cube Contents 1
John Lewis Stock Number 54600107 Name Chocoholic Cube 1. Atkins & Potts Milk Chocolate Dipper 100g 2. Chocca Mocca 100% Real Belgian Milk Drinking Chocolate 150g 3. Copperpot Milk Chocolate Coated Honeycomb 100g 4. Linden Lady Handmade White Chocolate Bar with Fruit & Nut 100g Contents 5. The Original Cake Company Chocolate Log Topped with Buttons & Choc Candy Beans 6. Frank's Luxury Double Choc Chunks Butter Bites 150g 7. Radfords Handmade West Country Chocolate & Caramel Fudge 113g 8. The Dormen Chocolate Peanuts 120g 9. Linden Lady Handmade 6 Luxury Chocolates 100g Net Quantity Individual product weights Manufacturer’s name and address Packer: Virginia Hayward Ltd Suitable for vegetarians Y/N Suitable for vegans Y/N Suitable for nut allergy sufferers Y/N Suitable for gluten allergy sufferers Y/N Suitable to wheat allergy sufferers Y/N Suitable for egg allergy sufferers Y/N Suitable for dairy allergy sufferers Y/N Suitable for soya allergy sufferers Y/N Free from alcohol Y/N Free from artificial flavours Y/N Free from artificial colours Y/N Refer to following sheets for individual product information. Product 1 Atkins & Potts Milk Chocolate Dipper 100g Sugar, Water, Glucose Syrup, Milk Chocolate (13%)(Sugar, Cocoa Butter, Whole Milk Powder, Cocoa Mass, Emulsifier: Soya Lecithin, Natural Vanilla), Butter Ingredients (Contains Milk), Skimmed Milk Powder, Cocoa Powder (2%), Emulsifier: Soya Lecithin, Sunflower Oil, Preservative: Potassium Sorbate, Stabiliser: Xanthan Gum. For allergens, see ingredients in bold May contain statements N/A Net Quantity 100g Country of Origin Made in the UK from ingredients of various origin. Store in a cool, dry place away from direct heat and Storage Instructions light. -

PREFERENCES AS BOUNDARY CONDITION of NUDGE EFFECTIVENESS E Potential of Nudges Under Empirical Investigation Tina A.G
PREFERENCES AS BOUNDARY CONDITION OF NUDGE OF EFFECTIVENESS : CONDITION BOUNDARY AS PREFERENCES PREFERENCES AS BOUNDARY CONDITION OF NUDGE EFFECTIVENESS e potential of nudges under empirical investigation e potential of nudges under empirical investigation empirical under nudges of potential e Tina A.G. Venema Dissertatiereeks Kurt Lewin Instituut 2019-06 Tina A.G. Venema PREFERENCES AS BOUNDARY CONDITION OF NUDGE EFFECTIVENESS The potential of nudges under empirical investigation Tina A.G. Venema ISBN: 978-90-393-7232-6 Layout & printing: Off Page, Amsterdam Cover illustration: Ashur - 99designs Copyright © 2019 by Annie Geziena Venema. All rights reserved. No part of this thesis may be reproduced, stored in a retrieval system or transmitted in any form or by any means without the prior written permission of the author. PREFERENCES AS BOUNDARY CONDITION OF NUDGE EFFECTIVENESS The potential of nudges under empirical investigation De effecitiviteit van nudges in relatie tot persoonlijke voorkeuren De potentie van nudges empirisch onderzocht (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de rector magnificus, prof.dr. H.R.B.M. Kummeling, ingevolge het besluit van het college voor promoties in het openbaar te verdedigen op vrijdag 17 januari 2020 des ochtends te 10.30 uur door Annie Geziena Venema geboren op 21 april 1991 te Bellingwolde Promotor: Prof. dr. D.T.D. De Ridder Copromotor: Dr. F.M. Kroese Dit proefschrift werd (mede) mogelijk gemaakt met financiële steun van NWO in de vorm van een TOP- subsidie voor het onderzoeksproject Welfare Improvement through Nudging Knowledge (407-13-030). -

Sep06 POSTER 091406.Indd
The National Cancer Institute at Frederick What is it? Where is it? Story on page 16. Dr. John Niederhuber Nominated as SEPTEMBER 2006 13th Director of NCI IN THIS ISSUE Dr. Elias Zerhouni, director of the National Institutes of Health, Science Today 3 announced last August that President Bush intends to name Dr. John Our Partners 4 Niederhuber the 13th director Off-Site Programs 6 of the National Cancer Institute. Dr. Niederhuber has been Acting Platinum Publications 8 Director since June and was Technology Transfer Branch 11 also NCI’s Deputy Director for Translational and Clinical Sciences. Environment, Health, and Safety In his e-mail message to NCI Program 12 employees, Dr. Zerhouni said, “A Where Are They Now? 15 renowned surgeon and research scientist, his career before coming The Poster Puzzler 16 to the NIH included positions at the University of Wisconsin School Poster People Profi le 17 of Medicine, Stanford University, FME Machine Shop 18 Johns Hopkins University School of Medicine, and the University Campus Improvement of Michigan. While at the Committee 19 University of Wisconsin, he directed Immediately before joining the NIH, Outreach and Special the University’s Comprehensive Dr. Niederhuber chaired the National Programs 20 Cancer Center supported by NCI. Cancer Advisory Board.” Ø Reporting In 21 2004 Chemistry Nobel Laureate Lectures Here Farmers’ Market 22 Frederick Employee Diversity Thirty years ago, not many people Recently, Dr. Hershko, a member of Team 24 realized that cancer can be caused by the the National Academy of Sciences, New Faces at NCI-Frederick 25 lack of degradation of an oncoprotein lectured here to an SRO crowd and or, on the other hand, by too rapid a simultaneous videocast to NCI- Special Events 26 degradation of a tumor suppressor Bethesda. -

CHAIR SUMMIT Master Class for Neuroscience Professional Development
#CHAIR2014 7TH ANNUAL CHAIR SUMMIT Master Class for Neuroscience Professional Development September 11 – 13, 2014 | Westin Tampa Harbour Island Sponsored by #CHAIR2014 Obesity And Food Addiction: A Global Concern Mark S. Gold, MD 17th University of Florida Distinguished Alumni Professor and Chairman University of Florida, Retired Gainesville, FL Mark S. Gold, MD Disclosures ● Chairman, Scientific Advisory Boards for Addiction & Psychiatry, Eating Disorders, Overeating, & Obesity for RiverMend Health and Wellspring Obesity Camps for Teens Mark S. Gold, MD Patent Disclosures Granted Patents & Sold ● Clonidine as an Opiate-Like Drug & Method of eliminating opiate withdrawal symptoms with clonidine in humans. #4,312,878 January 26, 1982 (See Gold, Redmond, Kleber, Lancet 1(8070)929-930, 1978. Patent Gold, Redmond, Kleber, +Aghajanian)—sold to Boerhinger ● Dopamine Depletion Hypothesis of Cocaine and Amphetamine Addiction & Brompocriptine –Dopamine Augmentation method of eliminating cocaine withdrawal. #731,102 and #791,188 ; #20, 1941.2,1986 US 7,611,858 BI April 20, 2007(Gold and Dackis Clin Ther 7(1):6-21, 1984 Dackis and Gold, Lancet 1(8438)1151-1152, 1985- –Sold to Novartis Mark S. Gold, MD Patent Disclosures Granted Patents & Licensed ● Detection of Cannabinoid Receptor Biomarkers and Uses Thereof Patent # US 7,611,858 BI April 20, 2007 ● Method and Apparatus for Detecting Environmental Smoke Exposure #7/052,468, May 30, 2006. ● Marker Detection Method and Apparatus to Monitor Drug Compliance #60/164,250, Filed November 8, 2000 ● Method and Apparatus for Detecting Illicit Substances #60/292,964 filed May 23, 2001 ● Methods and Apparatus for Therapeutic Drug Monitoring using Exhaled Breath #10/788,501 filed February 26, 2004 ● Novel Methods and Apparatus for Therapeutic Drug Monitoring Using Exhaled Breath # 10/788, 501, Filed February 26, 2004. -

Boundless Cures
Dr. Micozzi’s message of boundless cures taking what’s rightfully yours is already changing the• lives of people just like you… “You’re making a difference in so many lives,” writes Dan from Scottsdale, AZ An Insider’s Guide to Natural Healing in the New Millennium Guide to Natural An Insider’s Elaine Fisher wrote in to say… “Dr. Micozzi, thank you so much for the TRUTH and the diligence.” And Marlene couldn’t wait to tell Dr. Micozzi, “I treasure all of your information. I look forward to your posts like a• bear to honey. BLESS YOU.” STEP INSIDE A WORLD OF NEW CURES ONLINE! An Insider’s Guide WWW.DRMICOZZI.COM to Natural Healing Visit us online for more Insider information in the New Millennium and resources, including... • Breaking news on the latest developments in complementary and alternative health • Written to you by the Insider himself • • Better answers to today’s most threatening illnesses You’ll also find frequently asked questions, article archives, and an exclusive Subscribers-Only center M I where you can search and access back issues. c OZZI Marc S. Micozzi, M.D., Ph.D. OV2B000345 BOUNDLESS CURES An Insider’s Guide to Natural Healing in the New Millennium Marc S. Micozzi, M.D., Ph.D. ©Copyright 2015, OmniVista Health Media, L.L.C. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including recording, photocopying, or via a computerized or electric storage or retrieval system without permission granted in writing from the publisher. -

2010 Chronicle
undergraduate research CHRONICLEΎ̱̯̰̯ Program for Research Initiatives for Science Majors Welcome I’m delighted to introduce the first PRISM student research Chronicle. Now in its fourth year, the Program for Research Initiatives for Science Majors (PRISM) was founded to improve and expand opportunities for students to participate in faculty mentored research. These opportuni- ties not only provide student access to new equipment and procedures, they help build personal bonds between our students and faculty and help engage students in the practice of doing science. Thus, this Chronicle not only represents a culmination of the many achievements of our students, it is also a way to reach out to new students and demonstrate the diversity and depth of opportunities they have in our science programs. I am particularly pleased to highlight the research projects collected in this issue as they represent a significant step in the growth of PRISM. Five of the students featured in this Chronicle have decided to pursue their studies via graduate school – a record number for our program. Danielle Scimeca, featured on page 15, has gone on to an MD/PhD program at the University of Miami. Jason Quiñones, featured on page 13, is now in his first year of a PhD program in Pharmacology & Toxi- cology at the State University of New York at Stony Brook. And Tee- shavi Narayne, Katherine Reynoso, and Amanda Vasquez, featured on pages 11, 14, and 16, respectively, have each moved on to masters and doctorate programs within the CUNY system. Other students, such as Nazia Mahmood (page 9), have opted to work in teaching or labo- ratory work while they prepare for graduate school. -

The Willpower Instinct
Recommended by ECI Recommended by ECI Recommended by ECI Table of Contents Title Page Copyright Page Dedication Epigraph Introduction ONE - I Will, I Won’t, I Want: What Willpower Is, and Why It Matters TWO - The Willpower Instinct: Your Body Was Born to Resist Cheesecake THREE - Too Tired to Resist: Why Self-Control Is Like a Muscle FOUR - License to Sin: Why Being Good Gives Us Permission to Be Bad FIVE - The Brain’s Big Lie: Why We Mistake Wanting for Happiness SIX - What the Hell: How Feeling Bad Leads to Giving In SEVEN - Putting the Future on Sale: The Economics of Instant Gratification EIGHT - Infected! Why Willpower Is Contagious NINE - Don’t Read This Chapter: The Limits of “I Won’t” Power TEN - Final Thoughts Acknowledgements NOTES INDEX Recommended by ECI Published by the Penguin Group Penguin Group (USA) Inc., 375 Hudson Street, New York, New York 10014, USA • Penguin Group (Canada), 90 Eglinton Avenue East, Suite 700, Toronto, Ontario M4P 2Y3, Canada (a division of Pearson Penguin Canada Inc.) • Penguin Books Ltd, 80 Strand, London WC2R 0RL, England • Penguin Ireland, 25 St Stephen’s Green, Dublin 2, Ireland (a division of Penguin Books Ltd) • Penguin Group (Australia), 250 Camberwell Road, Camberwell, Victoria 3124, Australia (a division of Pearson Australia Group Pty Ltd) • Penguin Books India Pvt Ltd, 11 Community Centre, Panchsheel Park, New Delhi–110 017, India • Penguin Group (NZ), 67 Apollo Drive, Rosedale, North Shore 0632, New Zealand (a division of Pearson New Zealand Ltd) • Penguin Books (South Africa) (Pty) Ltd, 24 Sturdee Avenue, Rosebank, Johannesburg 2196, South Africa Penguin Books Ltd, Registered Offices: 80 Strand, London WC2R 0RL, England Copyright © 2012 by Kelly McGonigal, Ph.D. -

‚Food Addiction' – Addictive-Like Eating Behavior
Copyright! Reproduction and dissemination – also partial – applicable to all media only with Special | Food addiction written permission of Umschau Zeitschriftenverlag GmbH, Wiesbaden. Peer-reviewed | Manuscript received: January 31, 2017 | Revision accepted: April 13, 2017 © Antonio Balaguer soler/Hemera/Thinkstock ‚Food addiction‘ – addictive-like eating behavior The current state of research with the Yale Food Addiction Scale Carolin Hauck, Thomas Ellrott The construct of ,food addiction’ is increasingly a subject of focus both in public discussion and in scientifi c research. The concept of ,food addiction’ postulates a link between food intake and addiction. The aim of this article is to give nutrition experts a brief overview of the current scientifi c discussion, to provide an initial introduction to the complex topic of ,food addiction’, and to explain the possible clinical applications of the “Yale Food Addiction Scale” questionnaire (YFAS 2.0). 102 Ernaehrungs Umschau international | 6/2017 Copyright! Reproduction and dissemination – also partial – applicable to all media only with written permission of Umschau Zeitschriftenverlag GmbH, Wiesbaden. addiction’ is not included in DSM-5, Abstract however within specialist circles, it is being discussed as a new aspect of There are three methodical research approaches that are used to inves- addiction/eating disorders. The pur- tigate the construct of a ,food addiction’: animal studies, neurocogni- pose of this article is to provide an tive human studies using imaging methods, and questionnaire-based overview of the discussion as it now human studies using the Yale Food Addiction Scale (YFAS). The focus of stands, and in particular, to provide this article will be the current state of research using the YFAS 2.0. -

The Healthy Chocoholic
1 2018|HEALTHY CHOCOHOLIC | The Fit Soul by Amy Ramsey These statements have not been evaluated by the Food and Drug Administration. This is not intended to diagnose, treat, cure, or prevent any disease. Hi there! My name is Amy Ramsey and I am a Certified Health Coach through the Institute for Integrative Nutrition and a Personal Trainer through National Academy of Sports Medicine. I have a feeling you love chocolate as much as I do! I want to give you some delicious recipes that are good for you and a few tips to give you victory over the unhealthy chocolate addiction. I help women like you find lasting vitality and joy with life-changing nutrition, fun fitness, and endless inspiration to live a life filled with purpose. As a personal trainer and integrative nutrition health coach, I train busy women who want to fabulously toned body. I have taken my experience to create quick, express workouts exclusively for The Fit Soul programs, which combines fitness, nutrition, and community to help women heal their relationship with food, get fit, and have lasting results. This Fit Soul Approach is literally TRANSFORMING women's lives. You can be next! It’s my passion to teach women the health and wellness strategies they need to come to come ALIVE and THRIVE and truly live their best life! Love, Amy Ramsey 2 2018|HEALTHY CHOCOHOLIC | The Fit Soul by Amy Ramsey These statements have not been evaluated by the Food and Drug Administration. This is not intended to diagnose, treat, cure, or prevent any disease.