Better Captures Addictive-Like Eating Behavior
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevalence of Risk for Substance-Related and Behavioral Addictions Among University Students in Turkey
THE TURKISH JOURNAL ON ADDICTIONS www.addicta.com.tr ORIGINAL RESEARCH Prevalence of Risk for Substance-Related and Behavioral Addictions Among University Students in Turkey Esma Akpınar Aslan1 , Sedat Batmaz1 , Zekiye Çelikbaş1 , Oğuzhan Kılınçel2 , Gökben Hızlı Sayar3 , Hüseyin Ünübol3 1Department of Psychiatry, Tokat Gaziosmanpasa University School of Medicine, Tokat, Turkey 2Department of Child Development, İstanbul Gelişim University, İstanbul, Turkey 3Department of Psychiatry, Üsküdar University School of Medicine, İstanbul, Turkey ORCID iDs of the authors: E.A.A. 0000-0003-4714-6894; S.B. 0000-0003-0585-2184; Z.Ç. 0000-0003-4728-7304; O.K. 0000-0003-2988- 4631; G.H.S. 0000-0002-2514-5682; H.Ü. 0000-0003-4404-6062. Main Points • Men were found to be at higher risk for potential alcohol dependence, pathological gambling, gam- ing addiction, and sex/pornography addiction. • Mild nicotine addiction, problematic internet use and possible smartphone addiction, food addic- tion, and gaming addiction were higher in the age group of 18-24 years than in older students. • Screening for addiction risk in young people is crucial for effective prevention, early diagnosis, and treatment. • The screening programs and treatment targeting men and women, and different age groups need to be designed selectively. Abstract This study aims to investigate the prevalence of risk for substance-related and behavioral addictions among university students in Turkey. In total, 612 students were included in this study, and they completed an online questionnaire consisting of the Fagerström Test for Nicotine Dependence, the Alcohol Use Disorders Identifica- tion Test, the Drug Abuse Screening Test, the Smartphone Addiction Scale-Short Version, the Young’s Internet Addiction Test-Short Form, the South Oaks Gambling Screen, and the Burden of Behavioral Addiction Form. -

A Few Notes Hematopoiesis Innate Vs. Adaptive Immunity Main Stages
Molecular Disease Mechanisms 1 Molecular Disease Mechanisms 2 Adaptive Immunity A Few Notes Hematopoiesis Molecular Disease Mechanisms 3 Adaptive Immunity Molecular Disease Mechanisms 4 Adaptive Immunity Innate vs. Adaptive Immunity Cardinal Features of Adaptive Immune Response Main Stages of B Cell Development 2 Antwort 1 Antwort • production of 1011 cells per day Hi there, fellow biologist! I made these cards for the exam of the spring semester 2018 and thought I could improve my karma a bit by sharing them. They are probably not com- plete/won't entirely cover the next iteration of the course but they should be hella helpful. If you are wondering how i made these beauties, it's all made with LATEX- which I would highly recommend you to take a look at. And if you want to expand or change them or just for telling me what a wonderful person I am for sharing my hard work, send me an email at [email protected] and I'll send you the original LATEXfiles Cheers and best of luck, Pia PS: They have a lot of typos, sue me. 4 Antwort 3 Antwort • Specificity: mediated by specific receptors expressed on B cells (BcR) and Innate Immunity Adaptive Immunity T cells (TcR) lag time between exposure and max- immediate response (min, h) imal response (+/- 7 days) • Diversity: B and T cell receptors have great variability due to DNA rear- limited specificity - can distinguish highly antigen-specific rangements between different types of pathogens exposure results in no immunologic exposure results in immunologic • Clonal Expansion: B and T cells that recognize -

Effects of Nicotine on Homeostatic and Hedonic Components of Food Intake
235 1 A STOJAKOVIC and others Nicotine and energy 235:1 R13–R31 Review homeostasis Effects of nicotine on homeostatic and hedonic components of food intake Andrea Stojakovic1,2, Enma P Espinosa1,3, Osman T Farhad1 and Kabirullah Lutfy1 1Department of Pharmaceutical Sciences, College of Pharmacy, Western University of Health Sciences, Pomona, Correspondence California, USA should be addressed 2Mitochondrial Neurobiology and Therapeutics Laboratory, Mayo Clinic, Rochester, Minnesota, USA to K Lutfy 3Faculty of Medicine, School of Clinica Biochemistry, Pontifical Catholic University of Ecuador (PUCE), Quito, Ecuador Email [email protected] Abstract Chronic tobacco use leads to nicotine addiction that is characterized by exaggerated Key Words urges to use the drug despite the accompanying negative health and socioeconomic f nicotine burdens. Interestingly, nicotine users are found to be leaner than the general f food intake population. Review of the existing literature revealed that nicotine affects energy f obesity homeostasis and food consumption via altering the activity of neurons containing f orexigenic peptides orexigenic and anorexigenic peptides in the brain. Hypothalamus is one of the critical f anorexigenic peptides Endocrinology brain areas that regulates energy balance via the action of these neuropeptides. The of equilibrium between these two groups of peptides can be shifted by nicotine leading to decreased food intake and weight loss. The aim of this article is to review the existing Journal literature on the effect of nicotine on food intake and energy homeostasis and report on the changes that nicotine brings about in the level of these peptides and their receptors that may explain changes in food intake and body weight induced by nicotine. -

Short Note on Dark Side of Addiction
lism and D OPEN ACCESS Freely available online o ru h g o lc D A e p f e o n l d a e Journal of n r n c u e o J ISSN: 2329-6488 Alcoholism & Drug Dependence Editorial Short Note on Dark Side of Addiction Richard Samson* Department of Psychology, Faculty of Pharmacy, University of Michigan, 500 S State St, Ann Arbor, MI 48109, United States ABSTRACT Addiction is a biopsychosocial disorder characterized by repeated use of drugs, or repetitive engagement in a behavior such as gambling, despite harm to self and others. According to the "brain disease model of addiction," while a number of psychosocial factors contribute to the development and maintenance of addiction, a biological process that is induced by repeated exposure to an addictive stimulus is the core pathology that drives the development and maintenance of an addiction. Many scholars who study addiction argue that the brain disease model is incomplete and misleading. Emotions are "feeling" states and classic physiological emotive responses that are interpreted based on the history of the organism and the context. Motivation is a persistent state that leads to organized activity. Both are intervening variables and intimately related and have neural representations in the brain. The present thesis is that drugs of abuse elicit powerful emotions that can be interwoven conceptually into this framework. Keywords: Allostasis; Corticotropin-releasing factor; Dynorphin; Extended amygdala; Incentive salience; Opponent process INTRODUCTION clinical research in humans and preclinical studies involving ΔFosB have identified compulsive sexual activity – specifically, any form The brain disease model posits that addiction is a disorder of the of sexual intercourse – as an addiction (i.e., sexual addiction). -

Processed Food Addiction: a 21St Century Phenomenon 1
Processed Food Addiction: a 21st Century Phenomenon 1 Alcoholism– History Repeats: Processed Food Addiction, a 21st Century Phenomenon Karren-Lee Raymond PhD, Sally Hsueh-Chih Lai PhD, & Geoff Lovell PhD 1. Introduction The notion of processed food addiction has been represented in a myriad of ways, including food and addiction, chocolate addiction, eating addiction and not forgetting food processing (Brownell and Gold, 2014; Bruinsma and Taren, 1999; Hebebrand, et al., 2014; Moubarac, et al., 2014). Other terms used within the ‘food and addiction’ discourse include compulsive overeater, emotional binger, sugar addict, junk-foodie, binge eater, destructive relationships with food, and not to mention the slang labels for a person suffering from obesity such as fatty, fatso, piggie, gorger, tub, porker, and lard-ass. The ongoing lack of consensus among professionals and in the general community regarding appropriate terminology to describe the complications associated with processed food is similar to the debate on the use of the term alcoholism (Jelinek, 1960; Vaillant, 1983; White, 2014). In the latter part of the 18th Century and the early part of the 19th Century, Dipsomania was a term used to refer to a medical condition involving an uncontrollable craving for alcohol. This term is still mentioned in the WHO ICD- 10 classification as an alternative description for Alcohol Dependence Syndrome, episodic use F10.26 (World Health Organization, 2004). In following a similar path, Peínamania (Peína is the Greek word meaning hunger) could potentially be used as a description for a medical condition involving an uncontrollable craving for processed food. Furthermore, given the issue of weight stigmatisation (Meadows, and Daníelsdóttir 2016), with people who are overweight often perceived as having poor self-control Processed Food Addiction: a 21st Century Phenomenon 2 or ‘uncontrollable appetites’, processed food addicts could also be considered as ‘people with uncontrolled appetites’ hence, Peínamania. -
Food Addiction and the FA Solution: for Anyone Who Wants to Learn More
Food Addiction and the FA Solution: for anyone who wants to learn more. Food Addiction and the FA Solution | 1 hat makes some people continue to Weat when they are not hungry? Why are they unable to stick to a diet despite warnings from doctors and their own understanding of health and nutrition? Most people are familiar with the concept of alcoholism and drug addiction, but the idea that certain foods and quantities of foods can be addictive is only slowly gaining acceptance. This pamphlet is for anyone who wants to learn more about food addiction and the solution offered by Food Addicts in Recovery Anonymous (FA), a program based on the foodaddicts.org Twelve Steps of Alcoholics Anonymous (AA). Carl Lowe, Jr., MD, a fellow of the American College of Surgeons and a member of the American Society for Metabolic and Bariatric Surgery, shared the following observations: In my view, undeniably, food addiction is real. I see it every day. Looking for more ways to help my patients, I asked if I could sit in on an FA meeting. I stayed for a good while after the meeting ended, because I couldn’t pull myself away. I saw the changes FA was making in people’s lives, and I thought, This is exactly what my patients need. I refer all my patients to FA. I explain, “Right now, you have a relationship with food that is taking you down a road you don’t want to take. These people can help you.” FA seems to me to be a perfect solution. -

An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population
Medical University of South Carolina MEDICA MUSC Theses and Dissertations 2017 An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population Carley Garris Lovell Medical University of South Carolina Follow this and additional works at: https://medica-musc.researchcommons.org/theses Recommended Citation Lovell, Carley Garris, "An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population" (2017). MUSC Theses and Dissertations. 369. https://medica-musc.researchcommons.org/theses/369 This Dissertation is brought to you for free and open access by MEDICA. It has been accepted for inclusion in MUSC Theses and Dissertations by an authorized administrator of MEDICA. For more information, please contact [email protected]. ! ! An Exploratory Analysis of Psychological and Genetic Based Outcomes Related to Binge Drinking and Binge Eating Behaviors in a College-Age Population Carley Garris Lovell A dissertation submitted to the faculty of the Medical University of South Carolina in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the College of Nursing. September 15, 2017 ii! ! ACKNOWLEDGEMENTS I would like to thank my dissertation committee: Drs. Gayenell Magwood, Amy Adkins, Martina Mueller, and Dace Svikis. Dr. Magwood has been my advisor during my entire course of study, and her consistent encouragement and support as my chair allowed me to successfully complete my studies. She supplied valuable guidance throughout the journey, literally starting from day one. I appreciate her invaluable support. Dr. Mueller is one of the clearest thinkers that I have met, and I value her contributions to my study immensely. -

Surprise-Flavors-120620.Pdf
BANANA CREAM PIE CHOCOHOLIC CHUNK COCONUT CREAM PIE banana ice cream with marshmallow ripple deep dark chocolate ice cream coconut ice cream with marshmallow ripple, and sugar cookie gems with dark chocolate chips coconut flakes and sugar cookie gems BIRTHDAY CAKE CHOCOLATE COFFEE CHOCOLATE CHIP cake batter ice cream chocolate coffee flavored ice cream with a blue butter cream ripple and sprinkles flavored ice cream with chocolate chips BLUE MONSTER CHOCOLATE CHIP COOKIE DOUGH COTTON CANDY blue vanilla ice cream with Oreo ® cookies vanilla ice cream with cookie dough pieces cotton candy flavored ice cream and Nestle ® chocolate chip cookies and chocolate chips with mini chocolate rainbow chips BLUEBERRY COBBLER CHOCOLATE MALT WITH CARAMEL DEEP DISH APPLE PIE vanilla ice cream malted chocolate ice cream apple flavored ice cream with apple sauce ripple with blueberry ripple and sugar cookie gems with caramel ripple and cinnamon crunch pieces BUCKEYE CHOCOLATE PEANUT BUTTER BROWNIE EGGNOG peanut butter ice cream chocolate ice cream with Reese’s Peanut Butter® ripple eggnog with fudge ripple and buckeye candy pieces and brownie chunks flavored ice cream BUTTER PECAN CHOCOLATE PECAN GRAHAM CENTRAL STATION butter pecan flavored ice cream chocolate ice cream graham flavored ice cream with a graham cracker ripple with whole roasted, buttered and salted pecans with whole, roasted, buttered and salted pecans and chocolate covered crunchies CARAMEL APPLE CINNAMON ROLL HEAVENLY HASH sour apple flavored ice cream cake batter flavored ice cream chocolate -
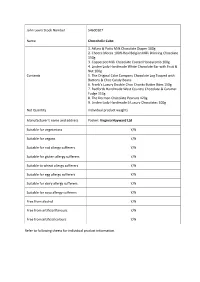
John Lewis Stock Number 54600107 Name Chocoholic Cube Contents 1
John Lewis Stock Number 54600107 Name Chocoholic Cube 1. Atkins & Potts Milk Chocolate Dipper 100g 2. Chocca Mocca 100% Real Belgian Milk Drinking Chocolate 150g 3. Copperpot Milk Chocolate Coated Honeycomb 100g 4. Linden Lady Handmade White Chocolate Bar with Fruit & Nut 100g Contents 5. The Original Cake Company Chocolate Log Topped with Buttons & Choc Candy Beans 6. Frank's Luxury Double Choc Chunks Butter Bites 150g 7. Radfords Handmade West Country Chocolate & Caramel Fudge 113g 8. The Dormen Chocolate Peanuts 120g 9. Linden Lady Handmade 6 Luxury Chocolates 100g Net Quantity Individual product weights Manufacturer’s name and address Packer: Virginia Hayward Ltd Suitable for vegetarians Y/N Suitable for vegans Y/N Suitable for nut allergy sufferers Y/N Suitable for gluten allergy sufferers Y/N Suitable to wheat allergy sufferers Y/N Suitable for egg allergy sufferers Y/N Suitable for dairy allergy sufferers Y/N Suitable for soya allergy sufferers Y/N Free from alcohol Y/N Free from artificial flavours Y/N Free from artificial colours Y/N Refer to following sheets for individual product information. Product 1 Atkins & Potts Milk Chocolate Dipper 100g Sugar, Water, Glucose Syrup, Milk Chocolate (13%)(Sugar, Cocoa Butter, Whole Milk Powder, Cocoa Mass, Emulsifier: Soya Lecithin, Natural Vanilla), Butter Ingredients (Contains Milk), Skimmed Milk Powder, Cocoa Powder (2%), Emulsifier: Soya Lecithin, Sunflower Oil, Preservative: Potassium Sorbate, Stabiliser: Xanthan Gum. For allergens, see ingredients in bold May contain statements N/A Net Quantity 100g Country of Origin Made in the UK from ingredients of various origin. Store in a cool, dry place away from direct heat and Storage Instructions light. -

PREFERENCES AS BOUNDARY CONDITION of NUDGE EFFECTIVENESS E Potential of Nudges Under Empirical Investigation Tina A.G
PREFERENCES AS BOUNDARY CONDITION OF NUDGE OF EFFECTIVENESS : CONDITION BOUNDARY AS PREFERENCES PREFERENCES AS BOUNDARY CONDITION OF NUDGE EFFECTIVENESS e potential of nudges under empirical investigation e potential of nudges under empirical investigation empirical under nudges of potential e Tina A.G. Venema Dissertatiereeks Kurt Lewin Instituut 2019-06 Tina A.G. Venema PREFERENCES AS BOUNDARY CONDITION OF NUDGE EFFECTIVENESS The potential of nudges under empirical investigation Tina A.G. Venema ISBN: 978-90-393-7232-6 Layout & printing: Off Page, Amsterdam Cover illustration: Ashur - 99designs Copyright © 2019 by Annie Geziena Venema. All rights reserved. No part of this thesis may be reproduced, stored in a retrieval system or transmitted in any form or by any means without the prior written permission of the author. PREFERENCES AS BOUNDARY CONDITION OF NUDGE EFFECTIVENESS The potential of nudges under empirical investigation De effecitiviteit van nudges in relatie tot persoonlijke voorkeuren De potentie van nudges empirisch onderzocht (met een samenvatting in het Nederlands) Proefschrift ter verkrijging van de graad van doctor aan de Universiteit Utrecht op gezag van de rector magnificus, prof.dr. H.R.B.M. Kummeling, ingevolge het besluit van het college voor promoties in het openbaar te verdedigen op vrijdag 17 januari 2020 des ochtends te 10.30 uur door Annie Geziena Venema geboren op 21 april 1991 te Bellingwolde Promotor: Prof. dr. D.T.D. De Ridder Copromotor: Dr. F.M. Kroese Dit proefschrift werd (mede) mogelijk gemaakt met financiële steun van NWO in de vorm van een TOP- subsidie voor het onderzoeksproject Welfare Improvement through Nudging Knowledge (407-13-030). -

The Impact of 'Food Addiction' on Food Policy
Curr Addict Rep DOI 10.1007/s40429-014-0015-x BEHAVIORAL ADDICTIONS (A GEARHARDT, SECTION EDITOR) The Impact of ‘Food Addiction’ on Food Policy Jennifer L. Pomeranz & Christina A. Roberto # Springer International Publishing AG 2014 Abstract As research on food and addiction evolves, it is alcohol in many ways, all three of these substances have important to consider how evidence for the addictive potential wreaked havoc on public health [1] and share important of certain foods could change public support for various food similarities [2•]. One key commonality between tobacco and policies designed to promote healthier choices. We draw alcohol is that both are considered to be addictive substances. lessons from the framing of addiction in the contexts of In contrast, it remains an open question whether certain foods tobacco and alcohol to discuss how an addiction frame for trigger an addictive process akin to known addictive sub- food might influence public perceptions and support for spe- stances. The research on food and addiction is too nascent to cific policies. We then evaluate the regulatory landscape in draw firm conclusions, but the conversation about whether tobacco and alcohol control to determine which policies may foods can be addictive is starting to permeate both the scien- be effective to protect the public against foods that may be tific community [3•, 4] and the lay public’s consciousness [5, harmful. We highlight several viable policy options that could 6]. The notion that foods can be addictive has been present in be implemented to protect public health from unhealthy food, popular culture for some time. -

Sep06 POSTER 091406.Indd
The National Cancer Institute at Frederick What is it? Where is it? Story on page 16. Dr. John Niederhuber Nominated as SEPTEMBER 2006 13th Director of NCI IN THIS ISSUE Dr. Elias Zerhouni, director of the National Institutes of Health, Science Today 3 announced last August that President Bush intends to name Dr. John Our Partners 4 Niederhuber the 13th director Off-Site Programs 6 of the National Cancer Institute. Dr. Niederhuber has been Acting Platinum Publications 8 Director since June and was Technology Transfer Branch 11 also NCI’s Deputy Director for Translational and Clinical Sciences. Environment, Health, and Safety In his e-mail message to NCI Program 12 employees, Dr. Zerhouni said, “A Where Are They Now? 15 renowned surgeon and research scientist, his career before coming The Poster Puzzler 16 to the NIH included positions at the University of Wisconsin School Poster People Profi le 17 of Medicine, Stanford University, FME Machine Shop 18 Johns Hopkins University School of Medicine, and the University Campus Improvement of Michigan. While at the Committee 19 University of Wisconsin, he directed Immediately before joining the NIH, Outreach and Special the University’s Comprehensive Dr. Niederhuber chaired the National Programs 20 Cancer Center supported by NCI. Cancer Advisory Board.” Ø Reporting In 21 2004 Chemistry Nobel Laureate Lectures Here Farmers’ Market 22 Frederick Employee Diversity Thirty years ago, not many people Recently, Dr. Hershko, a member of Team 24 realized that cancer can be caused by the the National Academy of Sciences, New Faces at NCI-Frederick 25 lack of degradation of an oncoprotein lectured here to an SRO crowd and or, on the other hand, by too rapid a simultaneous videocast to NCI- Special Events 26 degradation of a tumor suppressor Bethesda.