Chapter 5 Air Temperature and Humidity in the PBL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 8 Atmospheric Statics and Stability
Chapter 8 Atmospheric Statics and Stability 1. The Hydrostatic Equation • HydroSTATIC – dw/dt = 0! • Represents the balance between the upward directed pressure gradient force and downward directed gravity. ρ = const within this slab dp A=1 dz Force balance p-dp ρ p g d z upward pressure gradient force = downward force by gravity • p=F/A. A=1 m2, so upward force on bottom of slab is p, downward force on top is p-dp, so net upward force is dp. • Weight due to gravity is F=mg=ρgdz • Force balance: dp/dz = -ρg 2. Geopotential • Like potential energy. It is the work done on a parcel of air (per unit mass, to raise that parcel from the ground to a height z. • dφ ≡ gdz, so • Geopotential height – used as vertical coordinate often in synoptic meteorology. ≡ φ( 2 • Z z)/go (where go is 9.81 m/s ). • Note: Since gravity decreases with height (only slightly in troposphere), geopotential height Z will be a little less than actual height z. 3. The Hypsometric Equation and Thickness • Combining the equation for geopotential height with the ρ hydrostatic equation and the equation of state p = Rd Tv, • Integrating and assuming a mean virtual temp (so it can be a constant and pulled outside the integral), we get the hypsometric equation: • For a given mean virtual temperature, this equation allows for calculation of the thickness of the layer between 2 given pressure levels. • For two given pressure levels, the thickness is lower when the virtual temperature is lower, (ie., denser air). • Since thickness is readily calculated from radiosonde measurements, it provides an excellent forecasting tool. -

Comparison Between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels
AUGUST 2002 NOTES AND CORRESPONDENCE 885 Comparison between Observed Convective Cloud-Base Heights and Lifting Condensation Level for Two Different Lifted Parcels JEFFREY P. C RAVEN AND RYAN E. JEWELL NOAA/NWS/Storm Prediction Center, Norman, Oklahoma HAROLD E. BROOKS NOAA/National Severe Storms Laboratory, Norman, Oklahoma 6 January 2002 and 16 April 2002 ABSTRACT Approximately 400 Automated Surface Observing System (ASOS) observations of convective cloud-base heights at 2300 UTC were collected from April through August of 2001. These observations were compared with lifting condensation level (LCL) heights above ground level determined by 0000 UTC rawinsonde soundings from collocated upper-air sites. The LCL heights were calculated using both surface-based parcels (SBLCL) and mean-layer parcels (MLLCLÐusing mean temperature and dewpoint in lowest 100 hPa). The results show that the mean error for the MLLCL heights was substantially less than for SBLCL heights, with SBLCL heights consistently lower than observed cloud bases. These ®ndings suggest that the mean-layer parcel is likely more representative of the actual parcel associated with convective cloud development, which has implications for calculations of thermodynamic parameters such as convective available potential energy (CAPE) and convective inhibition. In addition, the median value of surface-based CAPE (SBCAPE) was more than 2 times that of the mean-layer CAPE (MLCAPE). Thus, caution is advised when considering surface-based thermodynamic indices, despite the assumed presence of a well-mixed afternoon boundary layer. 1. Introduction dry-adiabatic temperature pro®le (constant potential The lifting condensation level (LCL) has long been temperature in the mixed layer) and a moisture pro®le used to estimate boundary layer cloud heights (e.g., described by a constant mixing ratio. -

MSE3 Ch14 Thunderstorms
Chapter 14 Copyright © 2011, 2015 by Roland Stull. Meteorology for Scientists and Engineers, 3rd Ed. thunderstorms Contents Thunderstorms are among the most violent and difficult-to-predict weath- Thunderstorm Characteristics 481 er elements. Yet, thunderstorms can be Appearance 482 14 studied. They can be probed with radar and air- Clouds Associated with Thunderstorms 482 craft, and simulated in a laboratory or by computer. Cells & Evolution 484 They form in the air, and must obey the laws of fluid Thunderstorm Types & Organization 486 mechanics and thermodynamics. Basic Storms 486 Thunderstorms are also beautiful and majestic. Mesoscale Convective Systems 488 Supercell Thunderstorms 492 In thunderstorms, aesthetics and science merge, making them fascinating to study and chase. Thunderstorm Formation 496 Convective Conditions 496 Thunderstorm characteristics, formation, and Key Altitudes 496 forecasting are covered in this chapter. The next chapter covers thunderstorm hazards including High Humidity in the ABL 499 hail, gust fronts, lightning, and tornadoes. Instability, CAPE & Updrafts 503 CAPE 503 Updraft Velocity 508 Wind Shear in the Environment 509 Hodograph Basics 510 thunderstorm CharaCteristiCs Using Hodographs 514 Shear Across a Single Layer 514 Thunderstorms are convective clouds Mean Wind Shear Vector 514 with large vertical extent, often with tops near the Total Shear Magnitude 515 tropopause and bases near the top of the boundary Mean Environmental Wind (Normal Storm Mo- layer. Their official name is cumulonimbus (see tion) 516 the Clouds Chapter), for which the abbreviation is Supercell Storm Motion 518 Bulk Richardson Number 521 Cb. On weather maps the symbol represents thunderstorms, with a dot •, asterisk , or triangle Triggering vs. Convective Inhibition 522 * ∆ drawn just above the top of the symbol to indicate Convective Inhibition (CIN) 523 Trigger Mechanisms 525 rain, snow, or hail, respectively. -

Potential Vorticity
POTENTIAL VORTICITY Roger K. Smith March 3, 2003 Contents 1 Potential Vorticity Thinking - How might it help the fore- caster? 2 1.1Introduction............................ 2 1.2WhatisPV-thinking?...................... 4 1.3Examplesof‘PV-thinking’.................... 7 1.3.1 A thought-experiment for understanding tropical cy- clonemotion........................ 7 1.3.2 Kelvin-Helmholtz shear instability . ......... 9 1.3.3 Rossby wave propagation in a β-planechannel..... 12 1.4ThestructureofEPVintheatmosphere............ 13 1.4.1 Isentropicpotentialvorticitymaps........... 14 1.4.2 The vertical structure of upper-air PV anomalies . 18 2 A Potential Vorticity view of cyclogenesis 21 2.1PreliminaryIdeas......................... 21 2.2SurfacelayersofPV....................... 21 2.3Potentialvorticitygradientwaves................ 23 2.4 Baroclinic Instability . .................... 28 2.5 Applications to understanding cyclogenesis . ......... 30 3 Invertibility, iso-PV charts, diabatic and frictional effects. 33 3.1 Invertibility of EPV ........................ 33 3.2Iso-PVcharts........................... 33 3.3Diabaticandfrictionaleffects.................. 34 3.4Theeffectsofdiabaticheatingoncyclogenesis......... 36 3.5Thedemiseofcutofflowsandblockinganticyclones...... 36 3.6AdvantageofPVanalysisofcutofflows............. 37 3.7ThePVstructureoftropicalcyclones.............. 37 1 Chapter 1 Potential Vorticity Thinking - How might it help the forecaster? 1.1 Introduction A review paper on the applications of Potential Vorticity (PV-) concepts by Brian -

Thunderstorm Predictors and Their Forecast Skill for the Netherlands
Atmospheric Research 67–68 (2003) 273–299 www.elsevier.com/locate/atmos Thunderstorm predictors and their forecast skill for the Netherlands Alwin J. Haklander, Aarnout Van Delden* Institute for Marine and Atmospheric Sciences, Utrecht University, Princetonplein 5, 3584 CC Utrecht, The Netherlands Accepted 28 March 2003 Abstract Thirty-two different thunderstorm predictors, derived from rawinsonde observations, have been evaluated specifically for the Netherlands. For each of the 32 thunderstorm predictors, forecast skill as a function of the chosen threshold was determined, based on at least 10280 six-hourly rawinsonde observations at De Bilt. Thunderstorm activity was monitored by the Arrival Time Difference (ATD) lightning detection and location system from the UK Met Office. Confidence was gained in the ATD data by comparing them with hourly surface observations (thunder heard) for 4015 six-hour time intervals and six different detection radii around De Bilt. As an aside, we found that a detection radius of 20 km (the distance up to which thunder can usually be heard) yielded an optimum in the correlation between the observation and the detection of lightning activity. The dichotomous predictand was chosen to be any detected lightning activity within 100 km from De Bilt during the 6 h following a rawinsonde observation. According to the comparison of ATD data with present weather data, 95.5% of the observed thunderstorms at De Bilt were also detected within 100 km. By using verification parameters such as the True Skill Statistic (TSS) and the Heidke Skill Score (Heidke), optimal thresholds and relative forecast skill for all thunderstorm predictors have been evaluated. -

1 Module 4 Water Vapour in the Atmosphere 4.1 Statement of The
Module 4 Water Vapour in the Atmosphere 4.1 Statement of the General Meteorological Problem D. Brunt (1941) in his book Physical and Dynamical Meteorology has stated, “The main problem to be discussed in connection to the thermodynamics of the moist air is the variation of temperature produced by changes of pressure, which in the atmosphere are associated with vertical motion. When damp air ascends, it must eventually attain saturation, and further ascent produces condensation, at first in the form of water drops, and as snow in the later stages”. This statement of the problem emphasizes the role of vertical ascends in producing condensation of water vapour. However, several text books and papers discuss this problem on the assumption that products of condensation are carried with the ascending air current and the process is strictly reversible; meaning that if the damp air and water drops or snow are again brought downwards, the evaporation of water drops or snow uses up the same amount of latent heat as it was liberated by condensation on the upward path of the air. Another assumption is that the drops fall out as the damp air ascends but then the process is not reversible, and Von Bezold (1883) termed it as a pseudo-adiabatic process. It must be pointed out that if the products of condensation are retained in the ascending current, the mathematical treatment is easier in comparison to the pseudo-adiabatic case. There are four stages that can be discussed in connection to the ascent of moist air. (a) The air is saturated; (b) The air is saturated and contains water drops at a temperature above the freezing-point; (c) All the water drops freeze into ice at 0°C; (d) Saturated air and ice at temperatures below 0°C. -

Convective Cores in Continental and Oceanic Thunderstorms: Strength, Width, And
Convective Cores in Continental and Oceanic Thunderstorms: Strength, Width, and Dynamics THESIS Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University By Alexander Michael McCarthy, B.S. Graduate Program in Atmospheric Sciences The Ohio State University 2017 Master's Examination Committee: Jialin Lin, Advisor Jay Hobgood Bryan Mark Copyrighted by Alexander Michael McCarthy 2017 Abstract While aircraft penetration data of oceanic thunderstorms has been collected in several field campaigns between the 1970’s and 1990’s, the continental data they were compared to all stem from penetrations collected from the Thunderstorm Project in 1947. An analysis of an updated dataset where modern instruments allowed for more accurate measurements was used to make comparisons to prior oceanic field campaigns that used similar instrumentation and methodology. Comparisons of diameter and vertical velocity were found to be similar to previous findings. Cloud liquid water content magnitudes were found to be significantly greater over the oceans than over the continents, though the vertical profile of oceanic liquid water content showed a much more marked decrease with height above 4000 m than the continental profile, lending evidence that entrainment has a greater impact on oceanic convection than continental convection. The difference in buoyancy between vigorous continental and oceanic convection was investigated using a reversible CAPE calculation. It was found that for the strongest thunderstorms over the continents and oceans, oceanic CAPE values tended to be significantly higher than their continental counterparts. Alternatively, an irreversible CAPE calculation was used to investigate the role of entrainment in reducing buoyancy for continental and oceanic convection where it was found that entrainment played a greater role in diluting oceanic buoyancy than continental buoyancy. -

Stability Analysis, Page 1 Synoptic Meteorology I
Synoptic Meteorology I: Stability Analysis For Further Reading Most information contained within these lecture notes is drawn from Chapters 4 and 5 of “The Use of the Skew T, Log P Diagram in Analysis and Forecasting” by the Air Force Weather Agency, a PDF copy of which is available from the course website. Chapter 5 of Weather Analysis by D. Djurić provides further details about how stability may be assessed utilizing skew-T/ln-p diagrams. Why Do We Care About Stability? Simply put, we care about stability because it exerts a strong control on vertical motion – namely, ascent – and thus cloud and precipitation formation on the synoptic-scale or otherwise. We care about stability because rarely is the atmosphere ever absolutely stable or absolutely unstable, and thus we need to understand under what conditions the atmosphere is stable or unstable. We care about stability because the purpose of instability is to restore stability; atmospheric processes such as latent heat release act to consume the energy provided in the presence of instability. Before we can assess stability, however, we must first introduce a few additional concepts that we will later find beneficial, particularly when evaluating stability using skew-T diagrams. Stability-Related Concepts Convection Condensation Level The convection condensation level, or CCL, is the height or isobaric level to which an air parcel, if sufficiently heated from below, will rise adiabatically until it becomes saturated. The attribute “if sufficiently heated from below” gives rise to the convection portion of the CCL. Sufficiently strong heating of the Earth’s surface results in dry convection, which generates localized thermals that act to vertically transport energy. -

Chapter 2 Approximate Thermodynamics
Chapter 2 Approximate Thermodynamics 2.1 Atmosphere Various texts (Byers 1965, Wallace and Hobbs 2006) provide elementary treatments of at- mospheric thermodynamics, while Iribarne and Godson (1981) and Emanuel (1994) present more advanced treatments. We provide only an approximate treatment which is accept- able for idealized calculations, but must be replaced by a more accurate representation if quantitative comparisons with the real world are desired. 2.1.1 Dry entropy In an atmosphere without moisture the ideal gas law for dry air is p = R T (2.1) ρ d where p is the pressure, ρ is the air density, T is the absolute temperature, and Rd = R/md, R being the universal gas constant and md the molecular weight of dry air. If moisture is present there are minor modications to this equation, which we ignore here. The dry entropy per unit mass of air is sd = Cp ln(T/TR) − Rd ln(p/pR) (2.2) where Cp is the mass (not molar) specic heat of dry air at constant pressure, TR is a constant reference temperature (say 300 K), and pR is a constant reference pressure (say 1000 hPa). Recall that the specic heats at constant pressure and volume (Cv) are related to the gas constant by Cp − Cv = Rd. (2.3) A variable related to the dry entropy is the potential temperature θ, which is dened Rd/Cp θ = TR exp(sd/Cp) = T (pR/p) . (2.4) The potential temperature is the temperature air would have if it were compressed or ex- panded (without condensation of water) in a reversible adiabatic fashion to the reference pressure pR. -
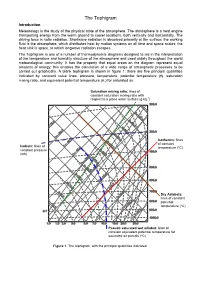
The Tephigram Introduction Meteorology Is the Study of the Physical State of the Atmosphere
The Tephigram Introduction Meteorology is the study of the physical state of the atmosphere. The atmosphere is a heat engine transporting energy from the warm ground to cooler locations, both vertically and horizontally. The driving force is solar radiation. Shortwave radiation is absorbed primarily at the surface; the working fluid is the atmosphere, which distributes heat by motion systems on all time and space scales; the heat sink is space, to which longwave radiation escapes. The Tephigram is one of a number of thermodynamic diagrams designed to aid in the interpretation of the temperature and humidity structure of the atmosphere and used widely throughout the world meteorological community. It has the property that equal areas on the diagram represent equal amounts of energy; this enables the calculation of a wide range of atmospheric processes to be carried out graphically. A blank tephigram is shown in figure 1; there are five principal quantities indicated by constant value lines: pressure, temperature, potential temperature (θ), saturation mixing ratio, and equivalent potential temperature (θe) for saturated air. Saturation mixing ratio: lines of constant saturation mixing ratio with respect to a plane water surface (g kg-1) Isotherms: lines of constant Isobars: lines of temperature (ºC) constant pressure (mb) Dry Adiabats: lines of constant potential temperature (ºC) Pseudo saturated wet adiabat: lines of constant equivalent potential temperature for saturated air parcels (ºC) Figure 1. The tephigram, with the principal quantities indicated. The principal axes of a tephigram are temperature and potential temperature; these are straight and perpendicular to each other, but rotated through about 45º anticlockwise so that lines of constant temperature run from bottom left to top right on the diagram. -

Dry Adiabatic Temperature Lapse Rate
ATMO 551a Fall 2010 Dry Adiabatic Temperature Lapse Rate As we discussed earlier in this class, a key feature of thick atmospheres (where thick means atmospheres with pressures greater than 100-200 mb) is temperature decreases with increasing altitude at higher pressures defining the troposphere of these planets. We want to understand why tropospheric temperatures systematically decrease with altitude and what the rate of decrease is. The first order explanation is the dry adiabatic lapse rate. An adiabatic process means no heat is exchanged in the process. For this to be the case, the process must be “fast” so that no heat is exchanged with the environment. So in the first law of thermodynamics, we can anticipate that we will set the dQ term equal to zero. To get at the rate at which temperature decreases with altitude in the troposphere, we need to introduce some atmospheric relation that defines a dependence on altitude. This relation is the hydrostatic relation we have discussed previously. In summary, the adiabatic lapse rate will emerge from combining the hydrostatic relation and the first law of thermodynamics with the heat transfer term, dQ, set to zero. The gravity side The hydrostatic relation is dP = −g ρ dz (1) which relates pressure to altitude. We rearrange this as dP −g dz = = α dP (2) € ρ where α = 1/ρ is known as the specific volume which is the volume per unit mass. This is the equation we will use in a moment in deriving the adiabatic lapse rate. Notice that € F dW dE g dz ≡ dΦ = g dz = g = g (3) m m m where Φ is known as the geopotential, Fg is the force of gravity and dWg is the work done by gravity. -

The Static Atmsophere Equation of State, Hydrostatic Balance, and the Hypsometric Equation
The Static Atmsophere Equation of State, Hydrostatic Balance, and the Hypsometric Equation Equation of State The atmosphere at any point can be described by its temperature, pressure, and density. These variables are related to each other through the Equation of State for dry air: pα = Rd T v (1) Where α is the specific volume (1/ρ), p = pressure, Tv = virtual temperature (K), and R is the gas constant for dry air (R = 287 J kg-1 K-1). The virtual temperature is the temperature air would have if we account for the moisture content of it. Above the surface, where moisture is scarce, Tv and T are nearly equal. Near the surface you can use the following equation to calculate Tv: TTv = (1+ 0.6078q) where q = specific humidity (T must be in K, q must be dimensionless). More on virtual temperature a little later….. We can also write the equation of state as: p= ρ Rd T v (2) The equation of state is also known as the “Ideal Gas Law”. The atmosphere is treated as an “Ideal Gas”, meaning that the molecules are considered to be of negligible size so that they exert no intermolecular forces. Hydrostatic Equation Except in smaller-scale systems such as an intense squall line, the vertical pressure gradient in the atmosphere is balanced by the gravity force. We call this the hydrostatic balance. dp = −ρg (3) dz Integrating (3) from a height z to the top of the atmosphere yields: ∞ p() z= ∫ ρ gdz (4) z This says that the pressure at any point in the atmosphere is equal to the weight of the air above it.