Overview of Development Pipeline Progress Status in Second Quarter of Fiscal Year 2017
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Review Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions Shilpa Gupta 1,†, David Gill 2,†, Austin Poole 2 and Neeraj Agarwal 2,* 1 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] 2 Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (D.G.); [email protected] (A.P.) * Correspondence: [email protected]; Tel.: +1-801-213-5658; Fax: +1-801-585-0124 † These authors contributed equally to this work. Academic Editor: Vita Golubovskaya Received: 14 December 2016; Accepted: 18 January 2017; Published: 27 January 2017 Abstract: Urothelial cancer of the bladder, renal pelvis, ureter, and other urinary organs is the fifth most common cancer in the United States, and systemic platinum-based chemotherapy remains the standard of care for first-line treatment of advanced/metastatic urothelial carcinoma (UC). Until recently, there were very limited options for patients who are refractory to chemotherapy, or do not tolerate chemotherapy due to toxicities and overall outcomes have remained very poor. While the role of immunotherapy was first established in non-muscle invasive bladder cancer in the 1970s, no systemic immunotherapy was approved for advanced disease until the recent approval of a programmed death ligand-1 (PD-L1) inhibitor, atezolizumab, in patients with advanced/metastatic UC who have progressed on platinum-containing regimens. This represents a significant milestone in this disease after a void of over 30 years. In addition to atezolizumab, a variety of checkpoint inhibitors have shown a significant activity in advanced/metastatic urothelial carcinoma and are expected to gain Food and Drug Administration (FDA) approval in the near future. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma
King’s Research Portal DOI: 10.3389/fimmu.2019.00453 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Khair, D. O., Bax, H. J., Mele, S., Crescioli, S., Pellizzari, G., Khiabany, A., Nakamura, M., Harris, R. J., French, E., Hoffmann, R. M., Williams, I. P., Cheung, K. K. A., Thair, B., Beales, C. T., Touizer, E., Signell, A. W., Tasnova, N. L., Spicer, J. F., Josephs, D. H., ... Karagiannis, S. N. (2019). Combining Immune Checkpoint Inhibitors: Established and Emerging Targets and Strategies to Improve Outcomes in Melanoma. Frontiers in Immunology , (MAR), [453]. https://doi.org/10.3389/fimmu.2019.00453 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. -
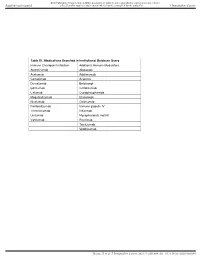
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories
bioRxiv preprint doi: https://doi.org/10.1101/572958; this version posted March 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories. Authors: Konrad Krawczyk1*, Matthew Raybould2, Aleksandr Kovaltsuk2, Charlotte M. Deane2 1 NaturalAntibody, Hamburg, Germany 2 Oxford University Department of Statistics, Oxford, UK *Correspondence to [email protected] Abstract: Recently it has become possible to query the great diversity of natural antibody repertoires using Next Generation Sequencing (NGS). These methods are capable of producing millions of sequences in a single experiment. Here we compare Clinical Stage Therapeutic antibodies to the ~1b sequences from 60 independent sequencing studies in the Observed Antibody Space Database. Of the 242 post Phase I antibodies, we find 16 with sequence identity matches of 95% or better for both heavy and light chains. There are also 54 perfect matches to therapeutic CDR-H3 regions in the NGS outputs, suggesting a nontrivial amount of convergence between naturally observed sequences and those developed artificially. This has potential implications for both the discovery of antibody therapeutics and the legal protection of commercial antibodies. Introduction Antibodies are proteins in jawed vertebrates that recognize noxious molecules (antigens) for elimination. An organism expresses millions of diverse antibodies to increase the chances that some of them will be able to bind the foreign antigen, initiating the adaptive immune response. -

The Evolving Landscape of `Next-Generation' Immune
European Journal of Cancer 117 (2019) 14e31 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.ejcancer.com Review The evolving landscape of ‘next-generation’ immune checkpoint inhibitors: A review Luca Mazzarella a, Bruno Achutti Duso a, Dario Trapani a,b, Carmen Belli a, Paolo D’Amico a,b, Emanuela Ferraro a,b, Giulia Viale a,b, Giuseppe Curigliano a,b,* a New Drugs and Early Drug Development for Innovative Therapies Division, IEO, European Institute of Oncology IRCCS, Milan, Italy b University of Milano, Department of Hematology and Hemato-Oncology, Italy Received 17 March 2019; received in revised form 23 April 2019; accepted 26 April 2019 Available online 21 June 2019 KEYWORDS Abstract ‘First-generation’ immune checkpoint inhibitors targeting Cytotoxic T-Lympho- Next generation cyte Antigen 4 (CTLA4) and Programmed death-ligand 1 (PD(L)1) have undoubtedly revolu- immune-checkpoints; tionised the treatment of multiple cancers in the advanced setting. Targeting signalling LAG3; pathways other than core inhibitory modules may strongly impact the outcome of the antitu- TIGIT; mour immune response. Drugs targeting these pathways (‘next-generation’ immune modula- TIM3; tors, NGIMs) constitute a major frontier in translational research and have generated 4-1BB; unprecedented scientific and financial investment. Here, we systematically reviewed published IDO1; literature, abstracts from major cancer conferences and pharma pipelines to identify NGIMs GITR; that have reached clinical development. We identified 107 molecules targeting 16 pathways, Colony stimulating which we classified into 6 groups according to function (inhibitory vs stimulatory) and cell factor-1 (CSF-1) of predominant expression (lymphoid, non-lymphoid and natural killer). -

An Ipsos Point of View
THE AMAZING RACE: NEXT-GEN IMMUNO- ONCOLOGY EDITION An Ipsos Point of View By Eric Blouin THE AMAZING RACE: After several false starts (remember OX40 NEXT-GEN IMMUNO- and the infamous IDO1?) and recent missteps (hello ICOS, we are looking at you!), two ONCOLOGY EDITION promising new immuno-oncology (IO) classes Summary could soon complement the reigning champions, In the search for next-generation immuno- anti-PD(L)1s. At ASCO 2020, Roche/Genentech oncology agents beyond the anti-PD-(L)1s, created buzz for tiragolumab (an anti-TIGIT two front-runners have finally emerged: candidate) with an update for CITYSCAPE, its BMS’ relatlimab (anti-LAG3) and Roche/ phase 2 NSCLC trial. More recently, BMS upped Genentech’s tiragolumab (anti-TIGIT). the ante by announcing positive results for The assets are complementary to anti- its RELATIVITY-047, a phase 2/3 trial for PD-(L)1s, potentially providing increased relatlimab, its anti-LAG-3 asset. efficacy (without incremental toxicities). In this article, we’ll analyze the strengths and Both companies have heavily invested in weaknesses of each class, and anticipate how broad clinical programs to expand beyond the assets’ initial lead indications. There the race might play out and what its impact is also intense competitive R&D activity. on the cancer landscape could be. Unlike most While relatlimab is likely to be approved contests, this one could have more than one first (based on having recently announced winner! a positive pivotal trial), its lead indication (metastatic melanoma) may not initially be a significant growth driver for BMS. In contrast, tiragolumab’s lead indication, 1L mNSCLC (PD-L1 high patients) holds greater immediate commercial benefit. -

Intratumoral Immunization: a New Paradigm for Cancer Therapy
Clinical Cancer Review Research Intratumoral Immunization: A New Paradigm for Cancer Therapy Aurelien Marabelle1, Holbrook Kohrt2, Christophe Caux1, and Ronald Levy2 Abstract Immune cell infiltration in the tumor microenvironment is of prognostic and therapeutic import. These immune cell subsets can be heterogeneous and are composed of mature antigen-presenting cells, helper and effector cytotoxic T cells, toleragenic dendritic cells, tumor-associated macrophages, and regulatory T cells, among other cell types. With the development of novel drugs that target the immune system rather than the cancer cells, the tumor immune microenvironment is not only prognostic for overall patient outcome, but also predictive for likelihood of response to these immune-targeted therapies. Such therapies aim to reverse the cancer immunotolerance and trigger an effective antitumor immune response. Two major families of immunostimulatory drugs are currently in clinical development: pattern recognition receptor agonists (PRRago) and immunostimulatory monoclonal antibodies (ISmAb). Despite their immune-targeted design, these agents have so far been developed clinically as if they were typical anticancer drugs. Here, we review the limitations of this conventional approach, specifically addressing the shortcomings of the usual schedules of intravenous infusions every 2 or 3 weeks. If the new modalities of immunotherapy target specific immune cells within the tumor microenvironment, it might be preferable to deliver them locally into the tumor rather than systemically. There is preclinical and clinical evidence that a therapeutic systemic antitumor immune response can be generated upon intratumoral immunomodulation. Moreover, pre- clinical results have shown that therapeutic synergy can be obtained by combining PRRagos and ISmAbs to the local tumor site. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Antibodies to Watch in 2021 Hélène Kaplona and Janice M
MABS 2021, VOL. 13, NO. 1, e1860476 (34 pages) https://doi.org/10.1080/19420862.2020.1860476 PERSPECTIVE Antibodies to watch in 2021 Hélène Kaplona and Janice M. Reichert b aInstitut De Recherches Internationales Servier, Translational Medicine Department, Suresnes, France; bThe Antibody Society, Inc., Framingham, MA, USA ABSTRACT ARTICLE HISTORY In this 12th annual installment of the Antibodies to Watch article series, we discuss key events in antibody Received 1 December 2020 therapeutics development that occurred in 2020 and forecast events that might occur in 2021. The Accepted 1 December 2020 coronavirus disease 2019 (COVID-19) pandemic posed an array of challenges and opportunities to the KEYWORDS healthcare system in 2020, and it will continue to do so in 2021. Remarkably, by late November 2020, two Antibody therapeutics; anti-SARS-CoV antibody products, bamlanivimab and the casirivimab and imdevimab cocktail, were cancer; COVID-19; Food and authorized for emergency use by the US Food and Drug Administration (FDA) and the repurposed Drug Administration; antibodies levilimab and itolizumab had been registered for emergency use as treatments for COVID-19 European Medicines Agency; in Russia and India, respectively. Despite the pandemic, 10 antibody therapeutics had been granted the immune-mediated disorders; first approval in the US or EU in 2020, as of November, and 2 more (tanezumab and margetuximab) may Sars-CoV-2 be granted approvals in December 2020.* In addition, prolgolimab and olokizumab had been granted first approvals in Russia and cetuximab saratolacan sodium was first approved in Japan. The number of approvals in 2021 may set a record, as marketing applications for 16 investigational antibody therapeutics are already undergoing regulatory review by either the FDA or the European Medicines Agency.