Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Predictive QSAR Tools to Aid in Early Process Development of Monoclonal Antibodies
Predictive QSAR tools to aid in early process development of monoclonal antibodies John Micael Andreas Karlberg Published work submitted to Newcastle University for the degree of Doctor of Philosophy in the School of Engineering November 2019 Abstract Monoclonal antibodies (mAbs) have become one of the fastest growing markets for diagnostic and therapeutic treatments over the last 30 years with a global sales revenue around $89 billion reported in 2017. A popular framework widely used in pharmaceutical industries for designing manufacturing processes for mAbs is Quality by Design (QbD) due to providing a structured and systematic approach in investigation and screening process parameters that might influence the product quality. However, due to the large number of product quality attributes (CQAs) and process parameters that exist in an mAb process platform, extensive investigation is needed to characterise their impact on the product quality which makes the process development costly and time consuming. There is thus an urgent need for methods and tools that can be used for early risk-based selection of critical product properties and process factors to reduce the number of potential factors that have to be investigated, thereby aiding in speeding up the process development and reduce costs. In this study, a framework for predictive model development based on Quantitative Structure- Activity Relationship (QSAR) modelling was developed to link structural features and properties of mAbs to Hydrophobic Interaction Chromatography (HIC) retention times and expressed mAb yield from HEK cells. Model development was based on a structured approach for incremental model refinement and evaluation that aided in increasing model performance until becoming acceptable in accordance to the OECD guidelines for QSAR models. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -
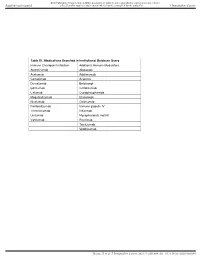
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

On the Horizon: Immuno-Oncology (I-O) Combinations
Immuno-Oncology (I-O) Combinations • Jeffrey A. Sosman, MD • Robert H. Lurie Comprehensive Cancer Center of Northwestern University The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 The Cancer–Immunity Cycle Daniel Chen and Ira Mellman Immunity, Volume 39, Issue 1, 2013, 1 - 10 Stimulatory and Inhibitory Factors in the Cancer-Immunity Cycle Each step of the Cancer-Immunity Cycle requires the coordination of numerous factors, both stimulatory and inhibitory in nature. Stimulatory factors shown in green promote immunity, ... Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Block other co-inhibitory: LAG3, TIM3, KIR, VISTA • Activate co-stimulatory: 4-1BB, OX-40, GITR, CD27, ICOS • Block inhibitory molecules- IDOi, TGFbi, CSF1Ri, anti-IL-6 or anti- IL-10 • Effect trafficking- anti-VEGF, CCL5, CXCR4i • Vaccines- TVEC- oncolytic virus, Neoantigen, other cellular • Adoptive Cellular therapy- TIL, CAR-T cells, TCR T-cells Where will Improvements come from? • Combinations: – Based on Template: anti-PD-1/PD-L1 or with anti-PD- 1/anti-CTLA-4 • Signal Inhibition, BRAF directed (BRAFi+MEKi), MEKi, PI3K inhibition (PTEN effects) • Cytokines- IL-2, IFN a,b,g,, Directed cytokines (FAP-IL-2v or CEA-IL-2v) • Epigenetic modulation- gene expression and EVR expression • Microbiome modification- fecal transplants • Chemotherapy other cytotoxics • Localized Irradiation SBRT, SRS T cells in Tumors Express Multiple Immunoinhibitory Receptors -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories
bioRxiv preprint doi: https://doi.org/10.1101/572958; this version posted March 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories. Authors: Konrad Krawczyk1*, Matthew Raybould2, Aleksandr Kovaltsuk2, Charlotte M. Deane2 1 NaturalAntibody, Hamburg, Germany 2 Oxford University Department of Statistics, Oxford, UK *Correspondence to [email protected] Abstract: Recently it has become possible to query the great diversity of natural antibody repertoires using Next Generation Sequencing (NGS). These methods are capable of producing millions of sequences in a single experiment. Here we compare Clinical Stage Therapeutic antibodies to the ~1b sequences from 60 independent sequencing studies in the Observed Antibody Space Database. Of the 242 post Phase I antibodies, we find 16 with sequence identity matches of 95% or better for both heavy and light chains. There are also 54 perfect matches to therapeutic CDR-H3 regions in the NGS outputs, suggesting a nontrivial amount of convergence between naturally observed sequences and those developed artificially. This has potential implications for both the discovery of antibody therapeutics and the legal protection of commercial antibodies. Introduction Antibodies are proteins in jawed vertebrates that recognize noxious molecules (antigens) for elimination. An organism expresses millions of diverse antibodies to increase the chances that some of them will be able to bind the foreign antigen, initiating the adaptive immune response. -

The Evolving Landscape of `Next-Generation' Immune
European Journal of Cancer 117 (2019) 14e31 Available online at www.sciencedirect.com ScienceDirect journal homepage: www.ejcancer.com Review The evolving landscape of ‘next-generation’ immune checkpoint inhibitors: A review Luca Mazzarella a, Bruno Achutti Duso a, Dario Trapani a,b, Carmen Belli a, Paolo D’Amico a,b, Emanuela Ferraro a,b, Giulia Viale a,b, Giuseppe Curigliano a,b,* a New Drugs and Early Drug Development for Innovative Therapies Division, IEO, European Institute of Oncology IRCCS, Milan, Italy b University of Milano, Department of Hematology and Hemato-Oncology, Italy Received 17 March 2019; received in revised form 23 April 2019; accepted 26 April 2019 Available online 21 June 2019 KEYWORDS Abstract ‘First-generation’ immune checkpoint inhibitors targeting Cytotoxic T-Lympho- Next generation cyte Antigen 4 (CTLA4) and Programmed death-ligand 1 (PD(L)1) have undoubtedly revolu- immune-checkpoints; tionised the treatment of multiple cancers in the advanced setting. Targeting signalling LAG3; pathways other than core inhibitory modules may strongly impact the outcome of the antitu- TIGIT; mour immune response. Drugs targeting these pathways (‘next-generation’ immune modula- TIM3; tors, NGIMs) constitute a major frontier in translational research and have generated 4-1BB; unprecedented scientific and financial investment. Here, we systematically reviewed published IDO1; literature, abstracts from major cancer conferences and pharma pipelines to identify NGIMs GITR; that have reached clinical development. We identified 107 molecules targeting 16 pathways, Colony stimulating which we classified into 6 groups according to function (inhibitory vs stimulatory) and cell factor-1 (CSF-1) of predominant expression (lymphoid, non-lymphoid and natural killer). -

Intratumoral Immunization: a New Paradigm for Cancer Therapy
Clinical Cancer Review Research Intratumoral Immunization: A New Paradigm for Cancer Therapy Aurelien Marabelle1, Holbrook Kohrt2, Christophe Caux1, and Ronald Levy2 Abstract Immune cell infiltration in the tumor microenvironment is of prognostic and therapeutic import. These immune cell subsets can be heterogeneous and are composed of mature antigen-presenting cells, helper and effector cytotoxic T cells, toleragenic dendritic cells, tumor-associated macrophages, and regulatory T cells, among other cell types. With the development of novel drugs that target the immune system rather than the cancer cells, the tumor immune microenvironment is not only prognostic for overall patient outcome, but also predictive for likelihood of response to these immune-targeted therapies. Such therapies aim to reverse the cancer immunotolerance and trigger an effective antitumor immune response. Two major families of immunostimulatory drugs are currently in clinical development: pattern recognition receptor agonists (PRRago) and immunostimulatory monoclonal antibodies (ISmAb). Despite their immune-targeted design, these agents have so far been developed clinically as if they were typical anticancer drugs. Here, we review the limitations of this conventional approach, specifically addressing the shortcomings of the usual schedules of intravenous infusions every 2 or 3 weeks. If the new modalities of immunotherapy target specific immune cells within the tumor microenvironment, it might be preferable to deliver them locally into the tumor rather than systemically. There is preclinical and clinical evidence that a therapeutic systemic antitumor immune response can be generated upon intratumoral immunomodulation. Moreover, pre- clinical results have shown that therapeutic synergy can be obtained by combining PRRagos and ISmAbs to the local tumor site. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Natural Killer Cell-Based Immunotherapy for Acute Myeloid Leukemia
Xu and Niu J Hematol Oncol (2020) 13:167 https://doi.org/10.1186/s13045-020-00996-x REVIEW Open Access Natural killer cell-based immunotherapy for acute myeloid leukemia Jing Xu and Ting Niu* Abstract Despite considerable progress has been achieved in the treatment of acute myeloid leukemia over the past decades, relapse remains a major problem. Novel therapeutic options aimed at attaining minimal residual disease-negative complete remission are expected to reduce the incidence of relapse and prolong survival. Natural killer cell-based immunotherapy is put forward as an option to tackle the unmet clinical needs. There have been an increasing num- ber of therapeutic dimensions ranging from adoptive NK cell transfer, chimeric antigen receptor-modifed NK cells, antibodies, cytokines to immunomodulatory drugs. In this review, we will summarize diferent forms of NK cell-based immunotherapy for AML based on preclinical investigations and clinical trials. Keywords: Acute myeloid leukemia, Natural killer cells, Immunotherapy, Adoptive NK cell transfer, Chimeric antigen receptor-modifed NK cells, Antibodies, Cytokines Background cells and substances in the immune system play pivotal Acute myeloid leukemia (AML) is a clinically and geneti- roles in detecting and destroying pathogen-infected or cally heterogeneous disease with unsatisfactory out- neoplastically transformed cells. But they become less comes. Over the last few years, considerable progress has potent in cancer elimination when malignant cells dis- been achieved in the treatment of AML with the devel- play the loss of antigenicity and/or immunogenicity and opment and implementation of new drugs [1, 2]. How- are surrounded by an immunosuppressive microenvi- ever, allogeneic hematopoietic cell transplantation (HCT) ronment [6]. -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

Study on Stabilization of Human Igg4 Antibodies
九州大学学術情報リポジトリ Kyushu University Institutional Repository Study on stabilization of human IgG4 antibodies 浪崎, 博史 https://doi.org/10.15017/2534401 出版情報:九州大学, 2019, 博士(創薬科学), 課程博士 バージョン: 権利関係: ヒト IgG4 抗体の安定化に関する研究 Study on stabilization of human IgG4 antibodies 2019 九州大学 大学院薬学府 臨床薬学部門 細胞生物薬学分野 浪崎 博史 1 略語表 CD; cluster of differentiation Ig; Immunoglobulin CDR; complementary determining region V; variable D; diversity J; joining RAG; recombination-activating gene RSS; recombination signal sequences TdT; terminal deoxynucleotidyl-transferase AID; activation induced cytidine deaminase BCR; B cell receptor TCR; T cell receptor Fab; fragment, antigen binding Fc; Fragment, Crystallizable VH; variable heavy CH; constant heavy VL; variable light CL; constant light CDC; complement-dependent cytotoxicity ADCC; antibody-dependent cellular cytotoxicity ADCP; antibody-dependent cellular phagocytosis NK; natural killer FcγR; Fc gamma receptor ITAM; immunoreceptor tyrosine-based activation motif 2 ITIM; immunoreceptor tyrosine-based inhibitory motif FcRn; neonatal Fc receptor PD-1; programmed cell death 1 PD-L1; programmed cell death –lignd1 VLA4; very late antigen-4 DNP; dinitrophenol ELISA; Enzyme-linked immunosorbent assay KD; dissociation constant SEC; size-exclusion chromatography DSF; differential scanning fluorimetry DSC; differential scanning calorimetry Tm; melting transition RU; resonance unit 3 目次 第一章 序論 p.5 1-1 背景 p.5 1-1-1 免疫グロブリンの種類および機能 p.5 1-1-2 免疫グロブリンの多様性獲得 p.7 1-1-3 ヒト IgG の構造および機能 p.9 1-1-4 抗体医薬の開発および承認状況 p.13 1-1-5 ヒト IgG4 の抗体医薬の研究開発状況 p.17 1-1-6 ヒト IgG サブクラスにおける安定性 p.21 1-2 引用文献 p.25 第二章 ヒト IgG4 抗体の低 pH 誘導性凝集体形成における アミノ酸改変による抑制効果に関する研究 p.30 2-1 要旨 p.30 2-2 研究背景 p.32 2-3 材料および方法 p.35 2-4 結果 p.43 2-5 考察 p.78 2-6 引用文献 p.87 謝辞 p.94 4 第一章 序論 1-1 背景 1-1-1 免疫グロブリンの種類および機能 免疫グロブリン(Immunoglobulin;Ig)は哺乳細胞に存在する B 細胞から産生される 糖タンパク質であり、外来抗原を認識して結合する機能を有する。Ig は主に血液中 および組織体液中に存在し、細菌やウイルスなどの外来抗原に結合することにより、 白血球またはマクロファージによる食作用、ならびに免疫細胞の結合による免疫作 用により生体防御に関わっている。ヒトの免疫グロブリンは、IgG, IgA, IgM, IgD お よび IgE の 5 つのクラスが存在し (Fig.