RWD – Acceptance by Regulatory Authorities RWD – Acceptance by Regulatory Authorities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions
Review Systemic Immunotherapy for Urothelial Cancer: Current Trends and Future Directions Shilpa Gupta 1,†, David Gill 2,†, Austin Poole 2 and Neeraj Agarwal 2,* 1 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455, USA; [email protected] 2 Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA; [email protected] (D.G.); [email protected] (A.P.) * Correspondence: [email protected]; Tel.: +1-801-213-5658; Fax: +1-801-585-0124 † These authors contributed equally to this work. Academic Editor: Vita Golubovskaya Received: 14 December 2016; Accepted: 18 January 2017; Published: 27 January 2017 Abstract: Urothelial cancer of the bladder, renal pelvis, ureter, and other urinary organs is the fifth most common cancer in the United States, and systemic platinum-based chemotherapy remains the standard of care for first-line treatment of advanced/metastatic urothelial carcinoma (UC). Until recently, there were very limited options for patients who are refractory to chemotherapy, or do not tolerate chemotherapy due to toxicities and overall outcomes have remained very poor. While the role of immunotherapy was first established in non-muscle invasive bladder cancer in the 1970s, no systemic immunotherapy was approved for advanced disease until the recent approval of a programmed death ligand-1 (PD-L1) inhibitor, atezolizumab, in patients with advanced/metastatic UC who have progressed on platinum-containing regimens. This represents a significant milestone in this disease after a void of over 30 years. In addition to atezolizumab, a variety of checkpoint inhibitors have shown a significant activity in advanced/metastatic urothelial carcinoma and are expected to gain Food and Drug Administration (FDA) approval in the near future. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -
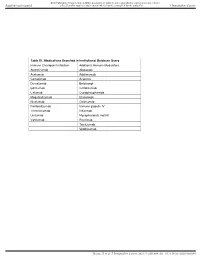
Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezol
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S1. Medications Searched in Institutional Database Query Immune Checkpoint Inhibitors Additional Immune Modulators Atezolizumab Abatacept Avelumab Adalimumab Cemiplimab Anakinra Durvalumab Belatacept Ipilimumab Certolizumab Lirilumab Cyclophosphamide Mogamulizumab Etanercept Nivolumab Golimumab Pembrolizumab Immune globulin IV Tremelimumab Infliximab Urelumab Mycophenolate mofetil Varlilumab Rituximab Tocilizumab Vedolizumab Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S2. Toxicity of additional immune modulators Treatment detail Toxicity Patient 1 day 18,32 Infliximab dosed day 59 P.aeruginosa, S.marcescens pneumonia; died Patient 2 day 9 Infliximab dosed day 44 Febrile neutropenia; P. aeruginosa SBP and day 26 Mycophenolate initiated sepsis; C. albicans fungemia; treated and discharged Patient 8 day 4,11 Infliximab dosed day 14 Disseminated HSV-1; died Patient 26 day 79-128 Infliximab dosed (x7) day 130 E. faecalis, P. aeruginosa bacteremia; died; day 81, 97 Cyclophosphamide dosed Fungal pneumonia on autopsy Beattie J, et al. J Immunother Cancer 2021; 9:e001884. doi: 10.1136/jitc-2020-001884 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) J Immunother Cancer Table S3. -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories
bioRxiv preprint doi: https://doi.org/10.1101/572958; this version posted March 10, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Title: Looking for Therapeutic Antibodies in Next Generation Sequencing Repositories. Authors: Konrad Krawczyk1*, Matthew Raybould2, Aleksandr Kovaltsuk2, Charlotte M. Deane2 1 NaturalAntibody, Hamburg, Germany 2 Oxford University Department of Statistics, Oxford, UK *Correspondence to [email protected] Abstract: Recently it has become possible to query the great diversity of natural antibody repertoires using Next Generation Sequencing (NGS). These methods are capable of producing millions of sequences in a single experiment. Here we compare Clinical Stage Therapeutic antibodies to the ~1b sequences from 60 independent sequencing studies in the Observed Antibody Space Database. Of the 242 post Phase I antibodies, we find 16 with sequence identity matches of 95% or better for both heavy and light chains. There are also 54 perfect matches to therapeutic CDR-H3 regions in the NGS outputs, suggesting a nontrivial amount of convergence between naturally observed sequences and those developed artificially. This has potential implications for both the discovery of antibody therapeutics and the legal protection of commercial antibodies. Introduction Antibodies are proteins in jawed vertebrates that recognize noxious molecules (antigens) for elimination. An organism expresses millions of diverse antibodies to increase the chances that some of them will be able to bind the foreign antigen, initiating the adaptive immune response. -

Intratumoral Immunization: a New Paradigm for Cancer Therapy
Clinical Cancer Review Research Intratumoral Immunization: A New Paradigm for Cancer Therapy Aurelien Marabelle1, Holbrook Kohrt2, Christophe Caux1, and Ronald Levy2 Abstract Immune cell infiltration in the tumor microenvironment is of prognostic and therapeutic import. These immune cell subsets can be heterogeneous and are composed of mature antigen-presenting cells, helper and effector cytotoxic T cells, toleragenic dendritic cells, tumor-associated macrophages, and regulatory T cells, among other cell types. With the development of novel drugs that target the immune system rather than the cancer cells, the tumor immune microenvironment is not only prognostic for overall patient outcome, but also predictive for likelihood of response to these immune-targeted therapies. Such therapies aim to reverse the cancer immunotolerance and trigger an effective antitumor immune response. Two major families of immunostimulatory drugs are currently in clinical development: pattern recognition receptor agonists (PRRago) and immunostimulatory monoclonal antibodies (ISmAb). Despite their immune-targeted design, these agents have so far been developed clinically as if they were typical anticancer drugs. Here, we review the limitations of this conventional approach, specifically addressing the shortcomings of the usual schedules of intravenous infusions every 2 or 3 weeks. If the new modalities of immunotherapy target specific immune cells within the tumor microenvironment, it might be preferable to deliver them locally into the tumor rather than systemically. There is preclinical and clinical evidence that a therapeutic systemic antitumor immune response can be generated upon intratumoral immunomodulation. Moreover, pre- clinical results have shown that therapeutic synergy can be obtained by combining PRRagos and ISmAbs to the local tumor site. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

Natural Killer Cell-Based Immunotherapy for Acute Myeloid Leukemia
Xu and Niu J Hematol Oncol (2020) 13:167 https://doi.org/10.1186/s13045-020-00996-x REVIEW Open Access Natural killer cell-based immunotherapy for acute myeloid leukemia Jing Xu and Ting Niu* Abstract Despite considerable progress has been achieved in the treatment of acute myeloid leukemia over the past decades, relapse remains a major problem. Novel therapeutic options aimed at attaining minimal residual disease-negative complete remission are expected to reduce the incidence of relapse and prolong survival. Natural killer cell-based immunotherapy is put forward as an option to tackle the unmet clinical needs. There have been an increasing num- ber of therapeutic dimensions ranging from adoptive NK cell transfer, chimeric antigen receptor-modifed NK cells, antibodies, cytokines to immunomodulatory drugs. In this review, we will summarize diferent forms of NK cell-based immunotherapy for AML based on preclinical investigations and clinical trials. Keywords: Acute myeloid leukemia, Natural killer cells, Immunotherapy, Adoptive NK cell transfer, Chimeric antigen receptor-modifed NK cells, Antibodies, Cytokines Background cells and substances in the immune system play pivotal Acute myeloid leukemia (AML) is a clinically and geneti- roles in detecting and destroying pathogen-infected or cally heterogeneous disease with unsatisfactory out- neoplastically transformed cells. But they become less comes. Over the last few years, considerable progress has potent in cancer elimination when malignant cells dis- been achieved in the treatment of AML with the devel- play the loss of antigenicity and/or immunogenicity and opment and implementation of new drugs [1, 2]. How- are surrounded by an immunosuppressive microenvi- ever, allogeneic hematopoietic cell transplantation (HCT) ronment [6]. -

WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/176089 Al 3 November 2016 (03.11.2016) P O P C T (51) International Patent Classification: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A01N 43/00 (2006.01) A61K 31/33 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US2016/028383 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 20 April 2016 (20.04.2016) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 62/154,426 29 April 2015 (29.04.2015) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (71) Applicant: KARDIATONOS, INC. [US/US]; 4909 DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, Lapeer Road, Metamora, Michigan 48455 (US). -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

A Abacavir Abacavirum Abakaviiri Abagovomab Abagovomabum
A abacavir abacavirum abakaviiri abagovomab abagovomabum abagovomabi abamectin abamectinum abamektiini abametapir abametapirum abametapiiri abanoquil abanoquilum abanokiili abaperidone abaperidonum abaperidoni abarelix abarelixum abareliksi abatacept abataceptum abatasepti abciximab abciximabum absiksimabi abecarnil abecarnilum abekarniili abediterol abediterolum abediteroli abetimus abetimusum abetimuusi abexinostat abexinostatum abeksinostaatti abicipar pegol abiciparum pegolum abisipaaripegoli abiraterone abirateronum abirateroni abitesartan abitesartanum abitesartaani ablukast ablukastum ablukasti abrilumab abrilumabum abrilumabi abrineurin abrineurinum abrineuriini abunidazol abunidazolum abunidatsoli acadesine acadesinum akadesiini acamprosate acamprosatum akamprosaatti acarbose acarbosum akarboosi acebrochol acebrocholum asebrokoli aceburic acid acidum aceburicum asebuurihappo acebutolol acebutololum asebutololi acecainide acecainidum asekainidi acecarbromal acecarbromalum asekarbromaali aceclidine aceclidinum aseklidiini aceclofenac aceclofenacum aseklofenaakki acedapsone acedapsonum asedapsoni acediasulfone sodium acediasulfonum natricum asediasulfoninatrium acefluranol acefluranolum asefluranoli acefurtiamine acefurtiaminum asefurtiamiini acefylline clofibrol acefyllinum clofibrolum asefylliiniklofibroli acefylline piperazine acefyllinum piperazinum asefylliinipiperatsiini aceglatone aceglatonum aseglatoni aceglutamide aceglutamidum aseglutamidi acemannan acemannanum asemannaani acemetacin acemetacinum asemetasiini aceneuramic -

NETTER, Jr., Robert, C. Et Al.; Dann, Dorf- (21) International Application
ll ( (51) International Patent Classification: (74) Agent: NETTER, Jr., Robert, C. et al.; Dann, Dorf- C07K 16/28 (2006.01) man, Herrell and Skillman, 1601 Market Street, Suite 2400, Philadelphia, PA 19103-2307 (US). (21) International Application Number: PCT/US2020/030354 (81) Designated States (unless otherwise indicated, for every kind of national protection av ailable) . AE, AG, AL, AM, (22) International Filing Date: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, 29 April 2020 (29.04.2020) CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, (25) Filing Language: English DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, (26) Publication Language: English KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (30) Priority Data: MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, 62/840,465 30 April 2019 (30.04.2019) US OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, ST, SV, SY, TH, TJ, TM, TN, TR, (71) Applicants: INSTITUTE FOR CANCER RESEARCH TT, TZ, UA, UG, US, UZ, VC, VN, WS, ZA, ZM, ZW. D/B/A THE RESEARCH INSTITUTE OF FOX CHASE CANCER CENTER [US/US]; 333 Cottman Av¬ (84) Designated States (unless otherwise indicated, for every enue, Philadelphia, PA 191 11-2497 (US). UNIVERSTIY kind of regional protection available) . ARIPO (BW, GH, OF KANSAS [US/US]; 245 Strong Hall, 1450 Jayhawk GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, Boulevard, Lawrence, KS 66045 (US).