Understanding the Thermal Stability and Environmental Sensitivity of Phycocyanin Using Spectroscopic and Modelling Tools
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View Article
Cronicon OPEN ACCESS EC Microbiology Review Article Spirulina Rising: Microalgae, Phyconutrients, and Oxidative Stress Mark F McCarty1 and Nicholas A Kerna2,3* 1Catalytic Longevity, USA 2SMC-Medical Research, Thailand 3First InterHealth Group, Thailand *Corresponding Author: Nicholas A Kerna, (mailing address) POB47 Phatphong, Suriwongse Road, Bangrak, Bangkok, Thailand 10500. Contact: [email protected] Received: August 22, 2019; Pubished: June 30, 2021 DOI: 10.31080/ecmi.2021.17.01135 Abstract Oxidative stress provokes the development of many common diseases and contributes to the aging process. Also, oxidative stress is a critical factor in common vascular disorders and type 2 diabetes. It plays a role in neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, amytrophic lateral sclerosis, multiple sclerosis, and cancer. Oxidative stress contributes to the healthy regulation of cell function. However, excessive oxidative stress results in pathological processes. Antioxidant vitamins have a limited from oxidative stress, and inhibits NOX. PhyCB, a component of the microalgae Spirulina, shares a similar structure with biliverdin, influence on oxidative stress as most oxidative stress results from the cellular production of superoxide. Bilirubin protects cells a biosynthetic precursor of bilirubin. Thus, the oral administration of PhyCB, phycocyanin, or whole Spirulina shows promise for preventing and or treating human disorders that have resulted from excessive oxidative stress. Keywords: Microalgae; Oxidative Stress; Phycocyanobilin; Phyconutrients; Singlet Oxygen; Spirulina; Superoxide Abbreviations DHA: Docosahexaenoic Acid; HO-1: Heme Oxygenase-1; O2: Oxygen; PhyCB: Phycocyanobilin Introduction To understand how phyconutrients, found abundantly in microalgae, can provide preventive and therapeutic effects, it is fundamental to understand how oxidative stress triggers or contributes to the development of many common diseases—and how microalgae, such as Spirulina can help inhibit oxidative stress in the human body. -

Myoglobin with Modified Tetrapyrrole Chromophores: Binding Specificity and Photochemistry ⁎ Stephanie Pröll A, Brigitte Wilhelm A, Bruno Robert B, Hugo Scheer A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1757 (2006) 750–763 www.elsevier.com/locate/bbabio Myoglobin with modified tetrapyrrole chromophores: Binding specificity and photochemistry ⁎ Stephanie Pröll a, Brigitte Wilhelm a, Bruno Robert b, Hugo Scheer a, a Department Biologie I-Botanik, Universität München, Menzingerstr, 67, 80638 München, Germany b Sections de Biophysique des Protéines et des Membranes, DBCM/CEA et URA CNRS 2096, C.E. Saclay, 91191 Gif (Yvette), France Received 2 August 2005; received in revised form 2 March 2006; accepted 28 March 2006 Available online 12 May 2006 Abstract Complexes were prepared of horse heart myoglobin with derivatives of (bacterio)chlorophylls and the linear tetrapyrrole, phycocyanobilin. Structural factors important for binding are (i) the presence of a central metal with open ligation site, which even induces binding of phycocyanobilin, and (ii) the absence of the hydrophobic esterifying alcohol, phytol. Binding is further modulated by the stereochemistry at the isocyclic ring. The binding pocket can act as a reaction chamber: with enolizable substrates, apo-myoglobin acts as a 132-epimerase converting, e.g., Zn-pheophorbide a' (132S) to a (132R). Light-induced reduction and oxidation of the bound pigments are accelerated as compared to solution. Some flexibility of the myoglobin is required for these reactions to occur; a nucleophile is required near the chromophores for photoreduction (Krasnovskii reaction), and oxygen for photooxidation. Oxidation of the bacteriochlorin in the complex and in aqueous solution continues in the dark. © 2006 Elsevier B.V. -

Phototrophic Pigment Production with Microalgae
Phototrophic pigment production with microalgae Kim J. M. Mulders Thesis committee Promotor Prof. Dr R.H. Wijffels Professor of Bioprocess Engineering Wageningen University Co-promotors Dr D.E. Martens Assistant professor, Bioprocess Engineering Group Wageningen University Dr P.P. Lamers Assistant professor, Bioprocess Engineering Group Wageningen University Other members Prof. Dr H. van Amerongen, Wageningen University Prof. Dr M.J.E.C. van der Maarel, University of Groningen Prof. Dr C. Vilchez Lobato, University of Huelva, Spain Dr S. Verseck, BASF Personal Care and Nutrition GmbH, Düsseldorf, Germany This research was conducted under the auspices of the Graduate School VLAG (Advanced studies in Food Technology, Agrobiotechnology, Nutrition and Health Sciences). Phototrophic pigment production with microalgae Kim J. M. Mulders Thesis submitted in fulfilment of the requirement for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr M.J. Kropff, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Friday 5 December 2014 at 11 p.m. in the Aula. K. J. M. Mulders Phototrophic pigment production with microalgae, 192 pages. PhD thesis, Wageningen University, Wageningen, NL (2014) With propositions, references and summaries in Dutch and English ISBN 978-94-6257-145-7 Abstract Microalgal pigments are regarded as natural alternatives for food colourants. To facilitate optimization of microalgae-based pigment production, this thesis aimed to obtain key insights in the pigment metabolism of phototrophic microalgae, with the main focus on secondary carotenoids. Different microalgal groups each possess their own set of primary pigments. Besides, a selected group of green algae (Chlorophytes) accumulate secondary pigments (secondary carotenoids) when exposed to oversaturating light conditions. -

Scalable Production of Biliverdin Ixα by Escherichia Coli Dong Chen1, Jason D Brown1, Yukie Kawasaki2, Jerry Bommer3 and Jon Y Takemoto1,2*
Chen et al. BMC Biotechnology 2012, 12:89 http://www.biomedcentral.com/1472-6750/12/89 RESEARCH ARTICLE Open Access Scalable production of biliverdin IXα by Escherichia coli Dong Chen1, Jason D Brown1, Yukie Kawasaki2, Jerry Bommer3 and Jon Y Takemoto1,2* Abstract Background: Biliverdin IXα is produced when heme undergoes reductive ring cleavage at the α-methene bridge catalyzed by heme oxygenase. It is subsequently reduced by biliverdin reductase to bilirubin IXα which is a potent endogenous antioxidant. Biliverdin IXα, through interaction with biliverdin reductase, also initiates signaling pathways leading to anti-inflammatory responses and suppression of cellular pro-inflammatory events. The use of biliverdin IXα as a cytoprotective therapeutic has been suggested, but its clinical development and use is currently limited by insufficient quantity, uncertain purity, and derivation from mammalian materials. To address these limitations, methods to produce, recover and purify biliverdin IXα from bacterial cultures of Escherichia coli were investigated and developed. Results: Recombinant E. coli strains BL21(HO1) and BL21(mHO1) expressing cyanobacterial heme oxygenase gene ho1 and a sequence modified version (mho1) optimized for E. coli expression, respectively, were constructed and shown to produce biliverdin IXα in batch and fed-batch bioreactor cultures. Strain BL21(mHO1) produced roughly twice the amount of biliverdin IXα than did strain BL21(HO1). Lactose either alone or in combination with glycerol supported consistent biliverdin IXα production by strain BL21(mHO1) (up to an average of 23. 5mg L-1 culture) in fed-batch mode and production by strain BL21 (HO1) in batch-mode was scalable to 100L bioreactor culture volumes. -

Biological Effects of Food Coloring in in Vivo and in Vitro Model Systems
foods Article Biological Effects of Food Coloring in In Vivo and In Vitro Model Systems Rocío Merinas-Amo, María Martínez-Jurado, Silvia Jurado-Güeto, Ángeles Alonso-Moraga and Tania Merinas-Amo * Department of Genetics, University of Córdoba, 14071 Córdoba, Spain; [email protected] (R.M.-A.); [email protected] (M.M.-J.); [email protected] (S.J.-G.); [email protected] (Á.A.-M.) * Correspondence: [email protected] Received: 18 April 2019; Accepted: 22 May 2019; Published: 24 May 2019 Abstract: (1) Background: The suitability of certain food colorings is nowadays in discussion because of the effects of these compounds on human health. For this reason, in the present work, the biological effects of six worldwide used food colorings (Riboflavin, Tartrazine, Carminic Acid, Erythrosine, Indigotine, and Brilliant Blue FCF) were analyzed using two model systems. (2) Methods: In vivo toxicity, antitoxicity, and longevity assays using the model organism Drosophila melanogaster and in vitro cytotoxicity, DNA fragmentation, and methylation status assays using HL-60 tumor human cell line were carried out. (3) Results: Our in vivo results showed safe effects in Drosophila for all the food coloring treatments, non-significant protective potential against an oxidative toxin, and different effects on the lifespan of flies. The in vitro results in HL-60 cells, showed that the tested food colorings increased tumor cell growth but did not induce any DNA damage or modifications in the DNA methylation status at their acceptable daily intake (ADI) concentrations. (4) Conclusions: From the in vivo and in vitro studies, these results would support the idea that a high chronic intake of food colorings throughout the entire life is not advisable. -

An Early Requisite for Efficient Photosynthesis in Cyanobacteria
EXCLI Journal 2015;14:268-289 – ISSN 1611-2156 Received: December 16, 2014, accepted: January 16, 2015, published: February 20, 2015 Original article: THE PHYCOBILISOMES: AN EARLY REQUISITE FOR EFFICIENT PHOTOSYNTHESIS IN CYANOBACTERIA Niraj Kumar Singh1,$, Ravi Raghav Sonani2,$, Rajesh Prasad Rastogi2,*, Datta Madamwar2,* 1 Shri A. N. Patel PG Institute (M. B. Patel Science College Campus), Anand, Sardargunj, Anand – 388001, Gujarat, India 2 BRD School of Biosciences, Sardar Patel Maidan, Vadtal Road, Post Box No. 39, Sardar Patel University, Vallabh Vidyanagar 388 120, Anand, Gujarat, India * Corresponding authors: Tel.: +91 02692 229380; fax: +91 02692 231042/236475; E-mail addresses: [email protected] (R.P. Rastogi), [email protected] (D. Madamwar) $ These authors have contributed equally. http://dx.doi.org/10.17179/excli2014-723 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/). ABSTRACT Cyanobacteria trap light energy by arrays of pigment molecules termed “phycobilisomes (PBSs)”, organized proximal to "reaction centers" at which chlorophyll perform the energy transduction steps with highest quantum efficiency. PBSs, composed of sequential assembly of various chromophorylated phycobiliproteins (PBPs), as well as nonchromophoric, basic and hydrophobic polypeptides called linkers. Atomic resolution structure of PBP is a heterodimer of two structurally related polypeptides but distinct specialised polypeptides- α and β, made up of seven alpha-helices each which played a crucial step in evolution of PBPs. PBPs carry out various light de- pendent responses such as complementary chromatic adaptation. The aim of this review is to summarize and discuss the recent progress in this field and to highlight the new and the questions that remain unresolved. -

JECFA), Which Was Held In
New_Cover_2016.pdf 1 26/04/2016 15:05:26 20 ISSN 1817-7077 COMPENDIUM OF FOOD ADDITIVE F A O J E C F A M o n o g r a p h s 22 SPECIFICATIONS 84th Meeting 2017 (JECFA), which was held in C M COMPENDIUM Y CM OF FOOD ADDITIVE MY CY SPECIFICATIONS CMY K Joint FAO/WHO Expert Committee on Food Additives 86th Meeting 2018 FAO / WHO FAO FAO JECFA Monographs 22 COMPENDIUM OF FOOD ADDITIVE SPECIFICATIONS Joint FAO/WHO Expert Committee on Food Additives 86th Meeting Geneva, 12 – 21 June 2018 Food and Agriculture Organization of the United Nations World Health Organization Geneva, 2018 II Required citation: FAO and WHO. 2018. Compendium of Food Additive Specifications. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 86th Meeting June 2018. FAO JECFA Monographs 22. Rome. 167 pp. Licence: CC BY-NC-SA 3.0 IGO. The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations (FAO) or the World Health Organization (WHO) concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by FAO or WHO in preference to others of a similar nature that are not mentioned. The views expressed in this information product are those of the author(s) and do not necessarily reflect the views or policies of FAO or WHO. -

Biomass and Phycocyanin from Oil and Natural Gas Extraction Produced Water Utilizing a Cyanobacteria Dominated Rotating Algal Biofilm Reactor (RABR)
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 8-2018 Biomass and Phycocyanin from Oil and Natural Gas Extraction Produced Water Utilizing a Cyanobacteria Dominated Rotating Algal Biofilm Reactor (RABR) Jonathan L. Wood Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Biological Engineering Commons Recommended Citation Wood, Jonathan L., "Biomass and Phycocyanin from Oil and Natural Gas Extraction Produced Water Utilizing a Cyanobacteria Dominated Rotating Algal Biofilm Reactor (RABR)" (2018). All Graduate Theses and Dissertations. 7073. https://digitalcommons.usu.edu/etd/7073 This Thesis is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. BIOMASS AND PHYCOCYANIN FROM OIL AND NATURAL GAS EXTRACTION PRODUCED WATER UTILIZING A CYANOBACTERIA DOMINATED ROTATING ALGAL BIOFILM REACTOR (RABR) by Jonathan L. Wood A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Biological Engineering Approved: ______________________ ____________________ Ronald. C. Sims, Ph.D. Jon Takemoto, Ph.D. Major Professor Committee Member ______________________ ____________________ Charles D. Miller, Ph.D. Mark R. McLellan, Ph.D. Committee Member Vice President for Research and Dean of the School of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2018 ii Copyright © Jonathan L. Wood 2018 All Rights Reserved iii ABSTRACT Biomass and phycocyanin from oil and natural gas extraction produced water utilizing a cyanobacteria dominated rotating algal biofilm reactor (RABR) by Jonathan L. -

The Loss of Green Color During Chlorophyll Degradation— a Prerequisite to Prevent Cell Death?
CORE Metadata, citation and similar papers at core.ac.uk Provided by Bern Open Repository and Information System (BORIS) Planta (2004) 219: 191–194 DOI 10.1007/s00425-004-1231-8 PROGRESS REPORT Stefan Ho¨rtensteiner The loss of green color during chlorophyll degradation— a prerequisite to prevent cell death? Received: 15 December 2003 / Accepted: 10 January 2004 / Published online: 8 April 2004 Ó Springer-Verlag 2004 Keywords Cell death Æ Chlorophyll degradation Æ product, red Chl catabolite (RCC). Thus, in vitro, RCC Non-fluorescent chlorophyll catabolite Æ does not accumulate in the absence of PaO. In addition, Pheophorbide a oxygenase Æ Red chlorophyll catabolite RCCR is sensitive to oxygen, although PaO requires O2 reductase Æ Senescence for incorporation into pheide a (Rodoni et al. 1997a; Wu¨thrich et al. 2000). PaO has been demonstrated to be a non-heme iron-containing monooxygenase, that spe- During plant senescence, chlorophyll (Chl) is degraded cifically introduces one oxygen atom of O at the a- to non-fluorescent Chl catabolites (NCCs; Fig. 1a). 2 methine bridge of pheide a (Ho¨rtensteiner et al. 1995, These linear tetrapyrroles accumulate in the vacuoles of 1998). In addition, PaO is specific for pheide a with senescing cells and, in many plant species, represent the pheide b inhibiting in a competitive manner (Ho¨rten- final products of Chl catabolism (Matile et al. 1988; steiner et al. 1995). PaO is located at the inner envelope Krautler 2003). Despite the billions of tons of Chl dis- ¨ membrane of senescing chloroplasts (Matile and Schel- appearing this way every year and the fascinating lenberg 1996). -

Evaluation of Certain Food Additives
WHO Technical Report Series 1014 Evaluation of certain food additives Eighty-sixth report of the Joint FAO/WHO Expert Committee on Food Additives The World Health Organization (WHO) was established in 1948 as a specialized agency of the United Nations serving as the directing and coordinating authority for international health matters and public health. One of WHO’s constitutional functions is to provide objective and reliable information and advice in the field of human health, a responsibility that it fulfils in part through its extensive programme of publications. The Organization seeks through its publications to support national health strategies and address the most pressing public health concerns of populations around the world. To respond to the needs of Member States at all levels of development, WHO publishes practical manuals, handbooks and training material for specific categories of health workers; internationally applicable guidelines and standards; reviews and analyses of health policies, programmes and research; and state-of-the-art consensus reports that offer technical advice and recommendations for decision-makers. These books are closely tied to the Organization’s priority activities, encompassing disease prevention and control, the development of equitable health systems based on primary health care, and health promotion for individuals and communities. Progress towards better health for all also demands the global dissemination and exchange of information that draws on the knowledge and experience of all WHO Member States and the collaboration of world leaders in public health and the biomedical sciences. To ensure the widest possible availability of authoritative information and guidance on health matters, WHO secures the broad international distribution of its publications and encourages their translation and adaptation. -
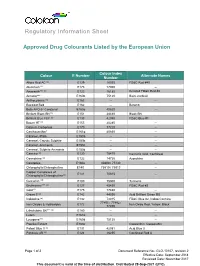
Regulatory Information Sheet
Regulatory Information Sheet Approved Drug Colourants Listed by the European Union Colour Index Colour E Number Alternate Names Number Allura Red AC (a) E129 16035 FD&C Red #40 Aluminum*** E173 77000 -- Amaranth*** (a) E123 16185 Delisted FD&C Red #2 Annatto*** E160b 75120 Bixin, norbixin Anthocyanins (a) E163 -- -- Beetroot Red E162 -- Betanin Beta APO-8´-Carotenal E160e 40820 -- Brilliant Black BN (a) E151 28440 Black BN Brilliant Blue FCF (a) E133 42090 FD&C Blue #1 Brown HT (a) E155 20285 -- Calcium Carbonate E170 77220 -- Canthaxanthin* E161g 40850 -- Caramel,-Plain E150a -- -- Caramel,-Caustic Sulphite E150b -- -- Caramel,-Ammonia E150c -- -- Caramel, Sulphite Ammonia E150d -- -- Carmine (a) E120 75470 Carminic Acid, Cochineal Carmoisine (a) E122 14720 Azorubine Carotenes E160a 40800 / 75130 -- Chlorophylls/Chlorophyllins E140 75810 / 75815 -- Copper Complexes of E141 75815 -- Chlorophylls/Chlorophyllins(a) Curcumin (a) E100 75300 Turmeric Erythrosine*** (a) E127 45430 FD&C Red #3 Gold*** E175 77480 -- Green S (a) E142 44090 Acid Brilliant Green BS Indigotine (a) E132 73015 FD&C Blue #2, Indigo Carmine 77491 / 77492 / Iron Oxides & Hydroxides E172 Iron Oxide Red, Yellow, Black 77499 Litholrubine BK*** (a) E180 -- -- Lutein E161b -- -- Lycopene*** E160d 75125 -- Paprika Extract E160c -- Capsanthin, Capsorubin Patent Blue V (a) E131 42051 Acid Blue 3 Ponceau 4R (a) E124 16255 Cochineal Red A Page 1 of 2 Document Reference No.: GLO-10107, revision 2 Effective Date: September 2014 Reviewed Date: November 2017 This document is valid at the time of distribution. Distributed 28-Sep-2021 (UTC) E Colour Index Colour Alternate Names Number Number Quinoline Yellow** (a) E104 47005 China Yellow Riboflavins (a) E101 -- -- Silver*** E174 -- -- Sunset Yellow FCF (a) E110 15985 FD&C Yellow #6, Orange Yellow S Tartrazine (a) E102 19140 FD&C Yellow #5 Titanium Dioxide E171 77891 -- Vegetable Carbon E153 77268:1 Carbo Medicinalis Vegetalis The above list is derived from Part B, List of All Additives, from Annex II to Regulation (EC) No 1333/2008 on food additives. -

Bacteriopheophytin G
Proc. Nati. Acad. Sci. USA Vol. 84, pp. 2570-2574, May 1987 Chemistry Bacteriopheophytin g: Properties and some speculations on a possible primary role for bacteriochlorophylls b and g in the biosynthesis of chlorophylls (photoisomerization/esterifying alcohol/electron spin resonance) T. J. MICHALSKI, J. E. HUNT, M. K. BOWMAN, U. SMITH, K. BARDEEN, H. GEST*, J. R. NORRIS, AND J. J. KATZ Chemistry Division, Argonne National Laboratory, Argonne, IL 60439 Contributed by J. J. Katz, January 14, 1987 ABSTRACT Bacteriopheophytin g and small amounts of characterized Bpheog by HPLC, and 252CF plasma desorp- bacteriochlorophyll g have been obtained in high purity from tion mass spectrometry (252Cf-PDMS). We have also studied the recently discovered photosynthetic bacterium Heliobacte- triplet Bpheog (3Bpheog) and the cation free radical BPheog' rium chlorum. Preparative methods and precautions in han- by ESR. Because of the high current interest in the structure dling these sensitive compounds are described. The compounds of bacterial photosynthetic reaction centers (6, 7), the reac- have been characterized by californium-252 plasma desorption tion center of H. chlorum has quickly attracted attention mass spectrometry, HPLC, visible absorption, and electron (8-10). The extreme sensitivity of BChlg to light and air was spin resonance spectroscopy. Our results agree with the struc- not adequately taken into consideration in the early reaction ture of bacteriochlorophyli g advanced by H. Brockmann and center work, and it will be necessary to do so in future A. Lipinski [(1983) Arch. Microbiol. 136, 17-191, with the reaction center research on this organism. exception that we find the esterifying alcohol to be farnesol and not geranylgeraniol as originally suggested.