Testing Protocol and Material Specifications for Basalt Fiber
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Performance Evaluation of 3-D Basalt Fiber Reinforced Concrete & Basalt
Highway IDEA Program Performance Evaluation of 3-D Basalt Fiber Reinforced Concrete & Basalt Rod Reinforced Concrete Final Report for Highway IDEA Project 45 Prepared by: V Ramakrishnan and Neeraj S. Tolmare, South Dakota School of Mines & Technology, Rapid City, SD Vladimir B. Brik, Research & Technology, Inc., Madison, WI November 1998 IDEA PROGRAM F'INAL REPORT Contract No. NCHRP-45 IDEA Program Transportation Resea¡ch Board National Research Council November 1998 PERFORMANCE EVALUATION OF 3.I) BASALT ITIBER REINFORCED CONCRETE & BASALT R:D RETNFORCED CONCRETE V. Rarnalaishnan Neeraj S. Tolmare South Dakota School of Mines & Technolory, Rapid City, SD Principal Investigator: Madimir B. Brik Research & Technology, lnc., Madison, WI INNOVATIONS DESERVING EXPLORATORY ANALYSIS (IDEA) PROGRAMS MANAGED BY THE TRANSPORTATION RESEARCH BOARD (TRB) This NCHRP-IDEA investigation was completed as part of the National Cooperative Highway Research Program (NCHRP). The NCHRP-IDEA program is one of the four IDEA programs managed by the Transportation Research Board (TRB) to foster innovations in highway and intermodal surface transportation systems. The other three IDEA program areas are Transit-IDEA, which focuses on products and results for transit practice, in support of the Transit Cooperative Research Program (TCRP), Safety-IDEA, which focuses on motor carrier safety practice, in support of the Federal Motor Carrier Safety Administration and Federal Railroad Administration, and High Speed Rail-IDEA (HSR), which focuses on products and results for high speed rail practice, in support of the Federal Railroad Administration. The four IDEA program areas are integrated to promote the development and testing of nontraditional and innovative concepts, methods, and technologies for surface transportation systems. -

Natural Fibers and Fiber-Based Materials in Biorefineries
Natural Fibers and Fiber-based Materials in Biorefineries Status Report 2018 This report was issued on behalf of IEA Bioenergy Task 42. It provides an overview of various fiber sources, their properties and their relevance in biorefineries. Their status in the scientific literature and market aspects are discussed. The report provides information for a broader audience about opportunities to sustainably add value to biorefineries by considerin g fiber applications as possible alternatives to other usage paths. IEA Bioenergy Task 42: December 2018 Natural Fibers and Fiber-based Materials in Biorefineries Status Report 2018 Report prepared by Julia Wenger, Tobias Stern, Josef-Peter Schöggl (University of Graz), René van Ree (Wageningen Food and Bio-based Research), Ugo De Corato, Isabella De Bari (ENEA), Geoff Bell (Microbiogen Australia Pty Ltd.), Heinz Stichnothe (Thünen Institute) With input from Jan van Dam, Martien van den Oever (Wageningen Food and Bio-based Research), Julia Graf (University of Graz), Henning Jørgensen (University of Copenhagen), Karin Fackler (Lenzing AG), Nicoletta Ravasio (CNR-ISTM), Michael Mandl (tbw research GesmbH), Borislava Kostova (formerly: U.S. Department of Energy) and many NTLs of IEA Bioenergy Task 42 in various discussions Disclaimer Whilst the information in this publication is derived from reliable sources, and reasonable care has been taken in its compilation, IEA Bioenergy, its Task42 Biorefinery and the authors of the publication cannot make any representation of warranty, expressed or implied, regarding the verity, accuracy, adequacy, or completeness of the information contained herein. IEA Bioenergy, its Task42 Biorefinery and the authors do not accept any liability towards the readers and users of the publication for any inaccuracy, error, or omission, regardless of the cause, or any damages resulting therefrom. -
All About Fibers
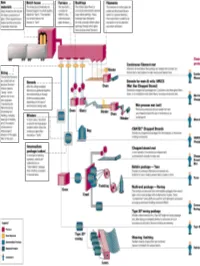
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Hybrid Woven Structures
HYBRID WOVEN STRUCTURES Hafsa Jamshaid, M.Sc. SUMMARY OF THE THESIS Title of the thesis: Woven Structures Author: Hafsa Jamshaid, M.Sc. Field of study: Textile Technics and Materials Engineering Mode of study: Full time Department: Material Engineering Supervisor: doc. Rajesh Mishra, Ph.D., B. Tech. Committee for the defense of the dissertation: chairman prof. Ing. Jiří Militký, CSc. FT TUL vice-chairwoman: Ing. Brigita Kolčavová Sirková, Ph.D. FT TUL prof. Ing. Lubomír Lapčík, Ph.D. (opponent) University of T. Baťa prof. Ing. Bohuslav Neckář, DrSc. FT TUL prof. Ing. Miroslav Václavík, CSc. (opponent) VÚTS, a. s., Liberec doc. Ing. Martin Bílek, Ph.D. (opponent) FS TUL doc. Dr. Ing. Dana Křemenáková FT TUL doc. Ing. Maroš Tunák, Ph.D. FT TUL Ing. Blanka Tomková, Ph.D. FT TUL The dissertation is available at the Dean's Office FT TUL. Liberec 2016 Abstract: With the advancement and continuing integration of composite materials and technology in today's modern industries, research in this field is becoming more and more significant. Basalt fibers are very promising materials due to their fire resistance related to magmatic origin, superior mechanical properties and relatively low cost. On the other hand, being a relatively new kind of fiber, they are still not studied extensively. There are very few indications in technical papers about their behavior after aging treatments. The current study investigates the possibility of using basalt with other types of yarns and consequently the effect of hybrid woven structure on load bearing capacity and durability. This thesis conveys a better insight into characteristics of Basalt fibers specifically, alongside commonly used fibers to design and develop hybrid woven fabrics for Textile Reinforced Concrete (TRC) materials. -

Fabrication and Characterization of Basalt/Kevlar/Aluminium Fiber Metal Laminates for Automobile Applications
International Journal of Materials Science ISSN 0973-4589 Volume 14, Number 1 (2019), pp. 1-9 © Research India Publications http://www.ripublication.com Fabrication and Characterization of Basalt/Kevlar/Aluminium Fiber Metal Laminates for Automobile Applications M.S. Santhosh1, R. Sasikumar2, T. Thangavel3, A. Pradeep4, K. Poovarasan5, S. Periyasamy6, T. Premkumar7 1 Junior Research Fellow, Selvam Composite Materials Research Laboratory, Tamilnadu, India. 2 Professor & Director Research, Selvam College of Technology, Tamilnadu, India. 3Assistant Professor, Mechanical Department, Paavai Engineering College, Tamilnadu, India. 4, 5, 6, 7 UG Scholars, Mechanical Department, Paavai Engineering College, Tamilnadu, India. Abstract Sandwich fiber metal laminates (FMLs) grabs significant growing attention among disparate engineering industries such as defense, aerospace and commercial vehicle manufacturers, due to its improved mechanical, thermal and electrical properties. Over the past few decades, FMLs were used as an impeccable identical for classical fiber composites like carbon and E-Glass. This prospective work interrogates mechanical behaviour of Basalt/Kevlar/AA 8090 reinforced FML fabricated by hand layup - compression moulding process. The low-velocity impact, flexural and tensile behavior of fabricated FMLs were calculated by various mechanical testings done as per ASTM standards. Fractured surface of the FML also analyzed by scanning electron microscopic images for understanding the fracture behavior of the proposed outcome. Keywords: Fiber metal laminate, Basalt, Kevlar, Al 8090, Flexural, Low velocity impact, compression moulding. 1. INTRODUCTION Fiber reinforced composites has a significant impact in production of engineering materials. It occupies huge percentage in the total fabrication due to its admirable mechanical properties like strength to weight ratio and cost-effectiveness. Recent researchers focusing on use fiber reinforced metal laminates for various automobile 2 M.S. -

Basalt Fiber and Its Applications
Journal of Textile Engineering & Fashion Technology Mini Review Open Access Basalt fiber and its applications Introduction Volume 1 Issue 6 - 2017 Technical textiles are new horizon for achievements in textile industry and it has become talk of the town in the recent past. Technical Hafsa Jamshaid textiles have a variety of applications and industries. Meeting end Department of Fabric Manufacturing, National Textile University, Pakistan product specification is a big challenge especially for industrial goods. Technical textiles are a rapidly developing and growing at a brisk pace Correspondence: Hafsa Jamshaid, National Textile in the textile industry. Textiles are replacing traditional materials in University, Faculty of Textile Engineering, Department of Fabric various sectors of the national economy. Growing environmental Manufacturing, Faisalabad, Pakistan, Email [email protected] awareness throughout the world has triggered a paradigm shift towards Received: April 18, 2017 | Published: June 01, 2017 designing materials compatible with the environment. The growing use of polymer composite materials in various field of technical textiles applications demands the development of products able to fulfill both technical and ever-stricter environmental requirements.1,2 Fiber reinforcements in composite material are generally used to improve the mechanical properties and environmental resistance when as any solvents, pigments or other hazardous materials are added. exposure to extreme environment takes place. The most common fiber Basalt fibers are environmental friendly as recycling of is much more reinforcement in resin is glass fiber .There is other types of fibers for efficient than glass fibers.9,10 Basalt fibers & fabrics are labeled as reinforcement such as carbon fiber and plastic fibers. -

Study on Gap Analysis
GAP ANALYSIS between the Performance Objectives Set Forth in the Framework Guidelines for Energy Efficiency Standards in Buildings and Current Energy Efficiency Standards and their Implementation in the Countries of South-Eastern and Eastern Europe, the Caucasus, Central Asia, and in the Russian Federation Source: Institute for Building Efficiency, WR ACKNOWLEDGEMENTS This report is prepared in the framework of the project “Enhancing National Capacities to Develop and Implement Energy Efficiency Standards for Buildings in the UNECE Region.” The report is prepared by the UNECE Secretariat. Nadejda Khamrakulova is the main author. Mohammed Abdul Mujeeb Khan has provided significant contributions. Oleg Dzioubinski, Scott Foster, and Igor Litvinyuk also contributed to this report. Valuable contributions at various stages of the research and the preparation of the report have been received from the following experts: • The UNECE Joint Task Force on Energy Efficiency Standards in Buildings; • The UNECE Group of Experts on Energy Efficiency; • Participants of the Workshop on Energy Efficiency Standards in Buildings and their Implementation in the UNECE region (Geneva and Chisinau (online), 9 April 2021); • In particular, the following contributors: Artan Leskoviku (Albania); Ani Rafyan, Andre Ohanian (Armenia); Andrei Miniankou (Belarus); Margalita Arabidze, Natalia Jamburia (Georgia); Mikhail Toropov (Kyrgyzstan); Sergiu Robu (Republic of Moldova); Kostiantyn Gura, Artem Makarov, Dana Galimuk (Ukraine); and Nizomiddin Rahmanov (Uzbekistan). -

Basalt Fiber Bar Reinforcement of Concrete Structures
REYKJAVÍK UNIVERSITY Basalt fiber bar Reinforcement of concrete structures Hannibal Ólafsson Eyþór Þórhallsson 2/11/2009 Basalt fiber bar Contents List of figures ............................................................................................................................. 2 Abstract ...................................................................................................................................... 3 Introduction ................................................................................................................................ 3 Properties of Basalt .................................................................................................................... 4 Basalt fiber ................................................................................................................................. 4 Fabrication .............................................................................................................................. 4 Basalt fibers compared to other FRP ...................................................................................... 5 Mechanical properties of the basalt fiber in this test .......................................................... 5 Alkali resistance ................................................................................................................. 6 Weathering resistance ........................................................................................................ 6 Thermal stability ............................................................................................................... -
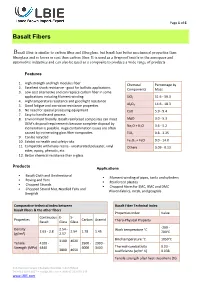
Basalt Fibers
Page 1 of 6 Basalt Fibers Basalt fiber is similar to carbon fiber and fiberglass, but basalt has better mechanical properties than fiberglass and is lower in cost than carbon fiber. It is used as a fireproof textile in the aerospace and automotive industries and can also be used as a composite to produce a wide range of products Features 1. High strength and high modulus fiber Chemical Percentage by 2. Excellent shock resistance - good for ballistic applications Components Mass 3. Low cost alternative and can replace carbon fiber in some applications including filament winding SiO2 51.6 - 59.3 4. High temperature resistance and good light resistance 5. Good fatigue and corrosion resistance properties Al2O3 14.6 - 18.3 6. No need for special processing equipment CaO 5.9 - 9.4 7. Easy to handle and process 8. Environment friendly. Basalt-reinforced composites can meet MgO 3.0 - 5.3 OEM's disposal requirements because complete disposal by Na O + K O 3.6 - 5.2 incineration is possible. Huge contamination issues are often 2 2 caused by incinerating glass fiber composites. TiO2 0.8 - 2.25 9. Can be recycled 10. Exhibit no health and safety risks Fe2O3 + FeO 9.0 - 14.0 11. Compatible with many resins - unsaturated polyester, vinyl Others 0.09 - 0.13 ester, epoxy, phenolic, etc. 12. Better chemical resistance than e-glass Products Applications Basalt Cloth and Unidirectional Filament winding of pipes, tanks and cylinders Roving and Yarn Reinforced plastics Chopped Strands Chopped fibers for SMC, BMC and DMC Chopped Strand Mat, Needled -

Mechanical Properties of a Unidirectional Basalt-Fiber/Epoxy Composite
Article Mechanical Properties of a Unidirectional Basalt-Fiber/Epoxy Composite David Plappert 1,* , Georg C. Ganzenmüller 1 , Michael May 2 and Samuel Beisel 1 1 Institute for Sustainable Systems Engineering, Albert-Ludwigs Universität Freiburg, 79100 Freiburg i. Br., Germany; [email protected] (G.C.G.); [email protected] (S.B.) 2 Fraunhofer Ernst-Mach-Institute for High-Speed Dynamics, EMI, 79100 Freiburg i. Br., Germany; [email protected] * Correspondence: [email protected] Received: 23 June 2020; Accepted: 27 July 2020; Published: 29 July 2020 Abstract: High-performance composites based on basalt fibers are becoming increasingly available. However, in comparison to traditional composites containing glass or carbon fibers, their mechanical properties are currently less well known. In particular, this is the case for laminates consisting of unidirectional plies of continuous basalt fibers in an epoxy polymer matrix. Here, we report a full quasi-static characterization of the properties of such a material. To this end, we investigate tension, compression, and shear specimens, cut from quality autoclave-cured basalt composites. Our findings indicate that, in terms of strength and stiffness, unidirectional basalt fiber composites are comparable to, or better than epoxy composites made from E-glass fibers. At the same time, basalt fiber composites combine low manufacturing costs with good recycling properties and are therefore well suited to a number of engineering applications. Keywords: basalt fibers; natural fibers; polymer-matrix composites (PMCs); mechanical properties; mechanical testing 1. Introduction Modern basalt fibers are a suitable high performance reinforcement for polymer composites. The mechanical properties of unidirectional basalt fiber reinforced polymers (BFRP) are similar to, or better than E-glass fiber reinforced polymers (GFRP)—elongation and stress at break are comparable [1] while their Young’s Modulus is higher by up to 35% [2]. -

Pdf X Iris XIV DBMC.Pdf
XIV DBMC 14 th International Conference on Durability of Building Materials and Components XIV DBMC – 14 th International Conference on Durability of Building Materials and Components, 29-31 May 2017, Ghent University, Belgium Published by RILEM Publications S.A.R.L. 4 avenue du Recteur Poincaré 75016 Paris - France Tel : + 33 1 42 24 64 46 Fax : + 33 9 70 29 51 20 http://www.rilem.net E-mail: [email protected] 2017 RILEM – Tous droits réservés. e-ISBN: 978-2-35158-159-9 Publisher's note : this book has been produced from electronic files provided by the individual con- tributors. The publisher makes no representation, express or implied, with regard to the accuracy of the information contained in this book and cannot accept any legal responsibility or liability for any errors or omissions that may be made. All titles published by RILEM Publications are under copyright protection; said copyrights being the property of their respective holders. All Rights Reserved. No part of any book may be reproduced or transmitted in any form or by any means, graphic, elec- tronic, or mechanical, including photocopying, recording, taping, or by any information storage or retrieval system, without the permission in writing from the publisher. RILEM, The International Union of Laboratories and Experts in Construction Materials, Sys- tems and Structures, is a non profit-making, non-governmental technical association whose vocation is to contribute to progress in the construction sciences, techniques and industries, essentially by means of the communication it fosters between research and practice. RILEM’s activity therefore aims at developing the knowledge of properties of materials and perfor- mance of structures, at defining the means for their assessment in laboratory and service con- ditions and at unifying measurement and testing methods used with this objective. -

Comparison of Basalt, Glass, and Carbon Fiber Composites Using the High Pressure Resin Transfer Molding Process
Comparison of Basalt, Glass, and Carbon Fiber Composites using the High Pressure Resin Transfer Molding Process Ian Swentek, Jeffrey Thompson Gleb Meirson, Vanja Ugresic, Frank Henning Fraunhofer Project Centre for Composites Research FPC @ Western A joint venture between: Western University, London, Ontario, Canada And Fraunhofer Gesellschaft, Munich, Germany; Contact: [email protected] Institute for Chemical Technology (ICT), Pfinztal, Germany www.eng.uwo.ca/fraunhofer RTM- Part 1 Two or more components are moved from separate heated tanks by high pressure pumps into the mix-head where they are mixed and injected into the mold. HP-RTM- Part 2 RTM mold Preform transfer into Resin injection and Opening and demolding mold, mold closing curing 15 sec 90 sec 15 sec Two minute cycle time Thanks to new resins this technology is now ready for high volume production structural applications Composite Manufacturing Dieffenbacher PreformCenter • Precise cutting of MD and UD fiber fabrics at any cutting angle • CNC cutting without paper underlay and vacuum foil • Continuous endless cutting to reduce material waste • Short cycle times Composite Manufacturing RTM Rimstar Thermo 8/4/8 with 2 Mixing Heads • Self-cleaning mixing head design • Separate mixing heads for epoxy and polyurethane systems • Internal mold release system can be used for third injection component • Precision dosing between 0.05 - 2.0 g/s • Mixing pressures between 60 and 180 bar • Resin flow rates: 20 - 120 g/s • Active pressure/flow monitoring Composite Manufacturing Dieffenbacher CompressPlus DCP-U 2500 • Parallel motion control system • Maximum closing force of 25,000 kN (using full parallel motion control force) • Minimum closing force of 250 kN • Rapid motion up to 800mm/s ram speed • Precision closing speeds up to 80mm/s at low force and 20mm/s at high force Introduction-Common Fiber Types Glass Fiber Basalt Fiber Carbon Fiber Use of basalt fiber has gained momentum as an alternative to traditional carbon and glass fiber.