The Neurophysiology of Backward Visual Masking: Information Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Levitis Et Al 2009
Animal Behaviour 78 (2009) 103–110 Contents lists available at ScienceDirect Animal Behaviour journal homepage: www.elsevier.com/locate/yanbe Behavioural biologists do not agree on what constitutes behaviour Daniel A. Levitis*, William Z. Lidicker, Jr, Glenn Freund Museum of Vertebrate Zoology and Department of Integrative Biology, University of California, Berkeley article info Behavioural biology is a major discipline within biology, centred on the key concept of ‘behaviour’. But Article history: how is ‘behaviour’ defined, and how should it be defined? We outline what characteristics we believe Received 10 February 2009 a scientific definition should have, and why we think it is important that a definition have these traits. Initial acceptance 12 March 2009 We then examine the range of available published definitions for behaviour. Finding no consensus, we Final acceptance 23 March 2009 present survey responses from 174 members of three behaviour-focused scientific societies as to their Published online 3 June 2009 understanding of the term. Here again, we find surprisingly widespread disagreement as to what MS. number: AE-09-00083 qualifies as behaviour. Respondents contradict themselves, each other and published definitions, indi- cating that they are using individually variable intuitive, rather than codified, meanings of ‘behaviour’. Keywords: We offer a new definition, based largely on survey responses: behaviour is the internally coordinated behaviour responses (actions or inactions) of whole living organisms (individuals or groups) to internal and/or definition external stimuli, excluding responses more easily understood as developmental changes. Finally, we level of organization philosophy of science discuss the usage, meanings and limitations of this definition. Ó 2009 The Association for the Study of Animal Behaviour. -

Hand on a Hot Stove
Hand on a Hot Stove Introduction: When You Put Your Hand on a Hot Stove Think about what happens if you accidentally place your hand on a hot stove. Use numbers 1-5 to place these statements in the order in which they happen. ____ You wave or shake your hand voluntarily to cool it. ____ Your arm moves to automatically move your hand away from the stove. ____ You feel pain in your hand. ____ You remember that you should not touch a hot stove. ____ You touch a hot stove. Life Sciences Learning Center 1 Copyright © 2013 by University of Rochester. All rights reserved. May be copied for classroom use Part 1: What is a reflex? Reflexes If you touch something that is very hot, your hand moves away quickly before you even feel the pain. You don’t have to think about it because the response is a reflex that does not involve the brain. A reflex is a rapid, unlearned, involuntary (automatic) response to a stimulus (change in the environment). Reflexes are responses that protect the body from potentially harmful events that require immediate action. They involve relatively few neurons (nerve cells) so that they can occur rapidly. There are a wide variety of reflexes that we experience every day such as sneezing, coughing, and blinking. We also automatically duck when an object is thrown at us, and our pupils automatically change size in response to light. These reflexes have evolved because they protect the body from potentially harmful events. Most reflexes protect people from injury or deal with things that require immediate action. -

Responses to Conflicting Stimuli in a Simple Stimulus–Response Pathway
2398 • The Journal of Neuroscience, February 11, 2015 • 35(6):2398–2406 Systems/Circuits Responses to Conflicting Stimuli in a Simple Stimulus–Response Pathway Pieter Laurens Baljon1 and XDaniel A. Wagenaar1,2 1California Institute of Technology, Division of Biology, Pasadena, California 91125, and 2University of Cincinnati, Department of Biological Sciences, Cincinnati, Ohio 45221 The “local bend response” of the medicinal leech (Hirudo verbana) is a stimulus–response pathway that enables the animal to bend away from a pressure stimulus applied anywhere along its body. The neuronal circuitry that supports this behavior has been well described, and its responses to individual stimuli are understood in quantitative detail. We probed the local bend system with pairs of electrical stimuli to sensory neurons that could not logically be interpreted as a single touch to the body wall and used multiple suction electrodes to record simultaneously the responses in large numbers of motor neurons. In all cases, responses lasted much longer than the stimuli that triggered them, implying the presence of some form of positive feedback loop to sustain the response. When stimuli were delivered simultaneously, the resulting motor neuron output could be described as an evenly weighted linear combination of the responses to the constituent stimuli. However, when stimuli were delivered sequentially, the second stimulus had greater impact on the motor neuron output, implying that the positive feedback in the system is not strong enough to render it immune to further input. Key words: invertebrate; neuronal circuits; sensory conflict; stimulus–response pathways Introduction Fortunately, lower animals also encounter sensory conflicts Sensory systems have traditionally been studied by neuroscien- and thus offer opportunities for studying circuits involved in tists one modality and one stimulus at a time. -

Appendix A: Glossary for Section 2.1 (PDF)
APPENDIX A GLOSSARY FOR SECTION 2.1 Sources: The Concise Columbia Encyclopedia. 1995. Columbia University Press; Solomon et al. 1993. Biology, Third Edition. Harcourt Brace Publishing astrocyte - a star-shaped cell, especially a neuroglial cell of nervous tissue. axon - the long, tubular extension of the neuron that conducts nerve impulses away from the cell body. blood-brain barrier - system of capillaries that regulates the movement of chemical substances, ions, and fluids in and out of the brain. central nervous system - the portion of the vertebrate nervous system consisting of the brain and spinal cord. cerebellum - the trilobed structure of the brain, lying posterior to the pons and medulla oblongata and inferior to the occipital lobes of the cerebral hemispheres, that is responsible for the regulation and coordination of complex voluntary muscular movement as well as the maintenance of posture and balance. cerebral cortex - the extensive outer layer of gray matter of the cerebral hemispheres, largely responsible for higher brain functions, including sensation, voluntary muscle movement, thought, reasoning, and memory. cerebrum - the large, rounded structure of the brain occupying most of the cranial cavity, divided into two cerebral hemispheres that are joined at the bottom by the corpus callosum. It controls and integrates motor, sensory, and higher mental functions, such as thought, reason, emotion, and memory. cognitive development - various mental tasks and processes (e.g. receiving, processing, storing, and retrieving information) that mediate between stimulus and response and determine problem-solving ability. demyelination - to destroy or remove the myelin sheath of (a nerve fiber), as through disease. dendrite - a branched protoplasmic extension of a nerve cell that conducts impulses from adjacent cells inward toward the cell body. -

Microbial Risk Assessment Guideline
EPA/100/J-12/001 USDA/FSIS/2012-001 MICROBIAL RISK ASSESSMENT GUIDELINE PATHOGENIC MICROORGANISMS WITH FOCUS ON FOOD AND WATER Prepared by the Interagency Microbiological Risk Assessment Guideline Workgroup July 2012 Microbial Risk Assessment Guideline Page ii DISCLAIMER This guideline document represents the current thinking of the workgroup on the topics addressed. It is not a regulation and does not confer any rights for or on any person and does not operate to bind USDA, EPA, any other federal agency, or the public. Further, this guideline is not intended to replace existing guidelines that are in use by agencies. The decision to apply methods and approaches in this guideline, either totally or in part, is left to the discretion of the individual department or agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. Environmental Protection Agency (EPA) (2012). Microbial Risk Assessment Guideline: Pathogenic Microorganisms with Focus on Food and Water. EPA/100/J-12/001 Microbial Risk Assessment Guideline Page iii TABLE OF CONTENTS Disclaimer .......................................................................................................................... ii Interagency Workgroup Members ................................................................................ vii Preface ............................................................................................................................. viii Abbreviations .................................................................................................................. -

Stimulus-Triggered Fate Conversion of Somatic Cells Into Pluripotency Haruko Obokata1,2,3, Teruhiko Wakayama3{, Yoshiki Sasai4, Koji Kojima1, Martin P
ARTICLE doi:10.1038/nature12968 Stimulus-triggered fate conversion of somatic cells into pluripotency Haruko Obokata1,2,3, Teruhiko Wakayama3{, Yoshiki Sasai4, Koji Kojima1, Martin P. Vacanti1,5, Hitoshi Niwa6, Masayuki Yamato7 & Charles A. Vacanti1 Here we report a unique cellular reprogramming phenomenon, called stimulus-triggered acquisition of pluripotency (STAP), which requires neither nuclear transfer nor the introduction of transcription factors. In STAP, strong external stimuli such as a transient low-pH stressor reprogrammed mammalian somatic cells, resulting in the generation of plu- ripotent cells. Through real-time imaging of STAP cells derived from purified lymphocytes, as well as gene rearrange- ment analysis, we found that committed somatic cells give rise to STAP cells by reprogramming rather than selection. STAP cells showed a substantial decrease in DNA methylation in the regulatory regions of pluripotency marker genes. Blastocyst injection showed that STAP cells efficiently contribute to chimaeric embryos and to offspring via germline transmission. We also demonstrate the derivation of robustly expandable pluripotent cell lines from STAP cells. Thus, our findings indicate that epigenetic fate determination of mammalian cells can be markedly converted ina context-dependent manner by strong environmental cues. In the canalization view of Waddington’s epigenetic landscape, fates (hereafter called LIF1B27 medium). Among the various perturbations, of somatic cells are progressively determined as cellular differentiation we were particularly interestedinlow-pHperturbations for tworeasons. proceeds, like going downhill. It is generally believed that reversal of First, as shown below, low-pH treatment turned out to be most effective differentiated status requires artificial physical or genetic manipulation for the induction of Oct4. -

History of Biology - Alberto M
BIOLOGICAL SCIENCE FUNDAMENTALS AND SYSTEMATICS – Vol. I – History of Biology - Alberto M. Simonetta HISTORY OF BIOLOGY Alberto M. Simonetta Dipartimento di Biologia Animale e Genetica, “L. Pardi,” University of Firenze, Italy Keywords: Biology, history, Antiquity, Middle ages, Renaissance, morphology, palaeontology, taxonomy, evolution, histology, embryology, genetics, ethology, ecology, pathology Contents 1. Introduction 2. Antiquity 3. The Medieval and Renaissance periods 4. The Development of Morphology 5. Paleontology 6. Taxonomy and Evolution 7. Histology, Reproduction, and Embryology 8. Physiology 9. Genetics 10. Ecology and Ethology 11. Pathology Bibliography Biographical Sketch Summary A short account is given of the development of biological sciences from their Greek origins to recent times. Biology as a pure science was the creation of Aristotle, but was abandoned shortly after his death. However, considerable advances relevant for medicine continued to be made until the end of classical times, in such fields as anatomy and botany. These developments are reviewed. After a long pause, both pure and applied research began anew in the thirteenth century, and developedUNESCO at an increasing pace therea fter.– However, EOLSS unlike astronomy and physics, which experienced a startling resurgence as soon as adequate mathematical methods and instruments became available, the development of biology was steady but slow until the appearance of Darwin’s revolutionary ideas about evolution brought about a fundamental shiftSAMPLE in the subject’s outlook. TheCHAPTERS efflorescence of biological sciences in the post-Darwinian period is outlined briefly. 1. Introduction To outline more than 2000 years of biology in a few pages is an extremely difficult endeavor as, quite apart from the complexities of both the subject itself and of the technical and theoretical approaches of various scholars, the development of scholars’ views, ideas, and researches forms an intricate network that cannot be fully disentangled in such a brief account. -
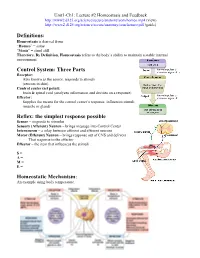
Three Parts Reflex: the Simplest Response Possible Homeostatic
Unit1-Ch1: Lecture #2 Homeostasis and Feedback http://www2.d125.org/science/secure/anatomy/som/homeo.mp4 (view) http://www2.d125.org/science/secure/anatomy/som/homeo.pdf (guide) Definitions: Homeostasis is derived from “Homeo” = same “Stasis” = stand still Therefore, By Definition, Homeostasis refers to the body’s ability to maintain a stable internal environment Control System: Three Parts Receptor: Also known as the sensor, responds to stimuli (sensors in skin) Control center (set point): brain & spinal cord (analyzes information and decides on a response) Effector: Supplies the means for the control center’s response, influences stimuli (muscle or gland) Reflex: the simplest response possible Sensor – responds to stimulus Sensory (Afferent) Neuron – brings message into Control Center Interneuron – a relay between afferent and efferent neurons Motor (Efferent) Neuron – brings response out of CNS and delivers That response to the effector Effector – the item that influences the stimuli S = A = M = E = Homeostatic Mechanism: An example using body temperature. Types of Feedback: Negative Feedback: Function: to maintain homeostasis Inhibitory: Produces an action opposite of the stimulus or initial conditions Stabilizing: returns conditions to the normal homeostatic balance Most Common: the majority of feedback mechanisms in the human body EXAMPLES: heart rate, temperature, breathing, glucose Positive Feedback: Not Homeostatic Amplifies the change that is occurring Destabilizing: disrupts homeostasis Stimulatory EXAMPLES: childbirth • Stimulus: pressure on cervix • Causes a release of oxytocin which causes smooth muscle contraction • Contractions create more pressure on cervix and cycle intensifies until baby is delivered • No more stimulus In positive feedback systems, the output enhances or exaggerates the original stimulus Example: Regulation of blood clotting . -

Leap 2025 Assessment Guide for Biology Posted August 2021 1
2021–2022 Assessment Guide for Biology This guide includes: Purpose Assessment Design Reporting Categories Test Administration Sample Test Items Resources PURPOSE This document is designed to assist Louisiana educators in understanding the LEAP 2025 Biology assessment. Introduction In order to create a more cohesive grades three through high school assessment system, the high school assessments transitioned from four-level to five- level tests. These new tests provide questions that have been reviewed by Louisiana educators to ensure their alignment to the Louisiana Student Standards and appropriateness for Louisiana students; consistency in graduation requirements; ability to measure the full range of student performance, including the performance of high- and low-performing students; and information for educators and parents about student readiness in Biology and whether students are “on track” for college and careers. For additional information about the high school assessment program, see the High School Assessment Frequently Asked Questions. New Vision for Science Standards and Assessments The Louisiana Student Standards for Science (LSS for Science) were created by over eighty content experts and educators with input from parents and teachers from across the state. Educators envisioned what students should know and be able to do to compete in our communities and created standards that would allow students to do so. The LSS for Science provide appropriate content for all grades or courses, maintain high expectations and create a logical connection of content across and within grades. The LSS for Science represent the knowledge and skills needed for students to successfully transition to postsecondary education and the workplace. The standards call for students to 1) apply content knowledge; 2) investigate, evaluate, and reason scientifically; and 3) connect ideas across disciplines. -

Adaptive Sampling in Sensory Physiology Jan Benda1, Tim Gollisch2, Christian K Machens3 and Andreas VM Herz1
CONEUR-503; NO OF PAGES 7 From response to stimulus: adaptive sampling in sensory physiology Jan Benda1, Tim Gollisch2, Christian K Machens3 and Andreas VM Herz1 Sensory systems extract behaviorally relevant information from in early vision [1], leading to an iterative process of data a continuous stream of complex high-dimensional input acquisition, data analysis, hypothesis generation, and new signals. Understanding the detailed dynamics and precise stimulus design. Each step in this process has traditionally neural code, even of a single neuron, is therefore a non-trivial been carried out separately. task. Automated closed-loop approaches that integrate data analysis in the experimental design ease the investigation of In this review, we discuss how one can use adaptive sensory systems in three directions: First, adaptive sampling sampling methods to speed up hypothesis-driven neuro- speeds up the data acquisition and thus increases the yield of science by analyzing neural responses while a cell is being an experiment. Second, model-driven stimulus exploration recorded and using the results of this analysis to deter- improves the quality of experimental data needed to mine the stimuli that should be presented next – all discriminate between alternative hypotheses. Third, during the same recording session. Of course, using their information-theoretic data analyses open up novel ways to intuition and expert knowledge, neurophysiologists often search for those stimuli that are most efficient in driving a given do just this – when they manually vary the parameters of a neuron in terms of its firing rate or coding quality. Examples visual grating to determine the receptive field of a neuron from different sensory systems show that, in all three or when they adjust the intensity of a sound stimulus to directions, substantial progress can be achieved once rapid measure the threshold curve of an auditory neuron. -

Lecture 1 Intro
1 Introduction Chapter 1 Introduction • Welcome to Our World • Thresholds and the Dawn of Psychophysics • Sensory Neuroscience and the Biology of Perception Introduction What do we mean by “Sensation & Perception?” • Sensation: The ability to detect a stimulus and, perhaps, to turn that detection into a private experience. • Perception: The act of giving meaning to a detected sensation. Sensation and perception are central to mental life. • Without them, how would we gain knowledge of the world? Introduction Psychologists typically study sensation and perception. • Also studied by biologists, computer scientists, medical doctors, neuroscientists, and many other fields Introduction The study of sensation and perception is a scientific pursuit and requires scientific methods. • Thresholds: Finding the limits of what can be perceived. • Scaling: Measuring private experience. • Signal detection theory: Measuring difficult decisions. • Sensory neuroscience: The biology of sensation and perception. • Neuroimaging: An image of the mind. Thresholds and the Dawn of Psychophysics Gustav Fechner (1801–1887) invented “psychophysics” and is often considered to be the true founder of experimental psychology. Fechner was an ambitious and hard- working young man who worked himself to the point of exhaustion. • Damaged his eyes by staring at the sun while performing vision experiments Figure 1.3 Gustav Fechner invented psychophysics and is thought by some to be the true founder of experimental psychology Thresholds and the Dawn of Psychophysics Fechner thought about the philosophical relationship between mind and matter. • Dualism: The mind has an existence separate from the material world of the body. • Materialism: The only thing that exists is matter, and that all things, including mind and consciousness, are the results of interactions between bits of matter. -

The Senses Reception of a Stimulus
The Senses eg. individual sensitivities, eg colorblindness we spend our lives in an ocean of sensory stimuli: ! unusual pathways, eg synesthesia light gravity electrical currents vibrations time our survival depends on our ability to perceive, interpret and respond to these signals Reception vs Perception Reception is the mechanism and structures involved in detecting and transmitting sensory information our body has millions of sensory receptors All sensory receptors are “connected to” our CNS by way of sensory neurons these neurons travel through the Cranial or Spinal nerves to the brain or spinal cord Perception is the conscious awareness of sensory stimuli is a higher level process of integration and interpretation depends on how the brain processes the signals it is receiving Human Anatomy & Physiology: The Senses; Ziser, Lecture Notes 2010.3 1 Human Anatomy & Physiology: The Senses; Ziser, Lecture Notes 2010.3 2 photoreceptor - light Reception of a Stimulus chemoreceptor - chemicals mechanoreceptor - bending, pressure, touch Reception of a sensation is determined by: thermoreceptor - temperature osmoreceptor – salt/water conc 1. Source of Sensory Stimuli baroreceptor - fluid pressure each sensory receptor is designed to transduce we can classify receptors by their location or only one kind of stimulus regardless of the source of the stimulus they respond to: how it is stimulated a. exteroceptors eg hard hit on head ! “see stars” near surface of body eg. spicy food monitor external environment eg. menthol cough drop most special senses 3. Density of Receptors & Size of Receptive b. visceroceptors (interoceptors) Field deep monitor internal environment 99% of receptors in body not evenly distributed over surface of body c.