Polysaccharide Diphtheria Toxoid Conjugate Vaccine Menactra®
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Research Brief March 2017 Publication #2017-16
Research Brief March 2017 Publication #2017-16 Flourishing From the Start: What Is It and How Can It Be Measured? Kristin Anderson Moore, PhD, Child Trends Christina D. Bethell, PhD, The Child and Adolescent Health Measurement Introduction Initiative, Johns Hopkins Bloomberg School of Every parent wants their child to flourish, and every community wants its Public Health children to thrive. It is not sufficient for children to avoid negative outcomes. Rather, from their earliest years, we should foster positive outcomes for David Murphey, PhD, children. Substantial evidence indicates that early investments to foster positive child development can reap large and lasting gains.1 But in order to Child Trends implement and sustain policies and programs that help children flourish, we need to accurately define, measure, and then monitor, “flourishing.”a Miranda Carver Martin, BA, Child Trends By comparing the available child development research literature with the data currently being collected by health researchers and other practitioners, Martha Beltz, BA, we have identified important gaps in our definition of flourishing.2 In formerly of Child Trends particular, the field lacks a set of brief, robust, and culturally sensitive measures of “thriving” constructs critical for young children.3 This is also true for measures of the promotive and protective factors that contribute to thriving. Even when measures do exist, there are serious concerns regarding their validity and utility. We instead recommend these high-priority measures of flourishing -

The Origin of the Peculiarities of the Vietnamese Alphabet André-Georges Haudricourt
The origin of the peculiarities of the Vietnamese alphabet André-Georges Haudricourt To cite this version: André-Georges Haudricourt. The origin of the peculiarities of the Vietnamese alphabet. Mon-Khmer Studies, 2010, 39, pp.89-104. halshs-00918824v2 HAL Id: halshs-00918824 https://halshs.archives-ouvertes.fr/halshs-00918824v2 Submitted on 17 Dec 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Published in Mon-Khmer Studies 39. 89–104 (2010). The origin of the peculiarities of the Vietnamese alphabet by André-Georges Haudricourt Translated by Alexis Michaud, LACITO-CNRS, France Originally published as: L’origine des particularités de l’alphabet vietnamien, Dân Việt Nam 3:61-68, 1949. Translator’s foreword André-Georges Haudricourt’s contribution to Southeast Asian studies is internationally acknowledged, witness the Haudricourt Festschrift (Suriya, Thomas and Suwilai 1985). However, many of Haudricourt’s works are not yet available to the English-reading public. A volume of the most important papers by André-Georges Haudricourt, translated by an international team of specialists, is currently in preparation. Its aim is to share with the English- speaking academic community Haudricourt’s seminal publications, many of which address issues in Southeast Asian languages, linguistics and social anthropology. -
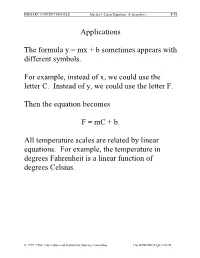
Applications the Formula Y = Mx + B Sometimes Appears with Different
PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-71 Applications The formula y = mx + b sometimes appears with different symbols. For example, instead of x, we could use the letter C. Instead of y, we could use the letter F. Then the equation becomes F = mC + b. All temperature scales are related by linear equations. For example, the temperature in degrees Fahrenheit is a linear function of degrees Celsius. © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-72 Basic Temperature Facts Water freezes at: 0°C, 32°F Water Boils at: 100°C, 212°F F • (100, 212) • (0, 32) C Can you solve for m and b in F = mC + b? © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-73 To find the equation relating Fahrenheit to Celsius we need m and b F – F m = 2 1 C2 – C1 212 – 32 m = 100 – 0 180 9 m = = 100 5 9 Therefore F = C + b 5 To find b, substitute the coordinates of either point. 9 32 = (0) + b 5 Therefore b = 32 Therefore the equation is 9 F = C + 32 5 Can you solve for C in terms of F? © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-74 Celsius in terms of Fahrenheit 9 F = C + 32 5 5 5 9 F = æ C +32ö 9 9è 5 ø 5 5 F = C + (32) 9 9 5 5 F – (32) = C 9 9 5 5 C = F – (32) 9 9 5 C = (F – 32) 9 Example: How many degrees Celsius is77°F? 5 C = (77 – 32) 9 5 C = (45) 9 C = 25° So 72°F = 25°C © 1999, CISC: Curriculum and Instruction Steering Committee The WINNING EQUATION PRIMARY CONTENT MODULE Algebra I -Linear Equations & Inequalities T-75 Standard 8 Algebra I, Grade 8 Standards Students understand the concept of parallel and perpendicular lines and how their slopes are related. -

Form Y-203-I, If You Had More Than One Job, Or If You Had a Job for Only Part of the Year
Department of Taxation and Finance Yonkers Nonresident Earnings Tax Return Y-203 For the full year January 1, 2020, through December 31, 2020, or fiscal year beginning and ending Name as shown on Form IT-201 or IT-203 Social Security number A Were you a Yonkers resident for any part of the taxable year? (mark an X in the appropriate box) Yes No (see instructions) (See the instructions for Form IT-201 or IT-203 for the definition of a resident.) If Yes: 1. Give period of Yonkers residence. From (mmddyyyy) to (mmddyyyy) 2. Are you reporting Yonkers resident income tax surcharge on your New York State return? ..................................................................................... Yes No (submit explanation) 3. You must complete and submit Form IT-360.1 (see instructions). B Did you or your spouse maintain an apartment or other living quarters in Yonkers during any part of the year?...................................................................................... Yes No If Yes, give address below and enter the number of days spent in Yonkers during 2020: days Address: C Are you reporting income from self-employment (on line 2 below)?......... Yes No If Yes, complete the following: Business name Business address Employer identification number Principal business activity Form of business: Sole proprietorship Partnership Other (explain) Calculation of nonresident earnings tax 1 Gross wages and other employee compensation (see instructions; if claiming an allocation, include amount from line 22) ............................................... 1 .00 2 Net earnings from self-employment (see instructions; if claiming an allocation, include amount from line 32; if a loss, write loss on line 2) ............................................................................................... 2 .00 3 Add lines 1 and 2 (if line 2 is a loss, enter amount from line 1) ........................................................... -

Summary Guide to Tetanus Prophylaxis in Routine Wound Management
Summary Guide to Tetanus Prophylaxis in Routine Wound Management ASSESS WOUND A clean, minor wound All other wounds (contaminated with dirt, feces, saliva, soil; puncture wounds; avulsions; wounds resulting from flying or crushing objects, animal bites, burns, frostbite) Has patient completed a primary Has patient completed a primary tetanus diphtheria series?1, 7 tetanus diphtheria series?1, 7 No/Unknown Yes No/Unknown Yes Administer vaccine today.2,3,4 Was the most recent Administer vaccine and Was the most Instruct patient to complete dose within the past tetanus immune gobulin recent dose within series per age-appropriate 10 years? (TIG) now.2,4,5,6,7 the past 5 years?7 vaccine schedule. No Yes No Yes Vaccine not needed today. Vaccine not needed today. Administer vaccine today.2,4 2,4 Patient should receive next Administer vaccine today. Patient should receive next Patient should receive next dose Patient should receive next dose dose at 10-year interval after dose at 10-year interval after per age-appropriate schedule. per age-appropriate schedule. last dose. last dose. 1 A primary series consists of a minimum of 3 doses of tetanus- and diphtheria- 4 Tdap* is preferred for persons 11 through 64 years of age if using Adacel* or 10 years of containing vaccine (DTaP/DTP/Tdap/DT/Td). age and older if using Boostrix* who have never received Tdap. Td is preferred to tetanus 2 Age-appropriate vaccine: toxoid (TT) for persons 7 through 9 years, 65 years and older, or who have received a • DTaP for infants and children 6 weeks up to 7 years of age (or DT pediatric if Tdap previously. -

Vaccines 101 the Very Last Day That I Was a Pediatric Resident. Um, Many
Vaccines 101 The very last day that I was a pediatric resident. Um, many years ago, a toddler walked into the emergency room and uh, and progressively got sicker and sicker. That's Dr. Katherine Edwards, a world expert in pediatric infectious disease in vaccinology. She's also a professor of pediatrics at Vanderbilt university and she's been working on vaccines for 40 years. I did a spinal tap on her and realized that she had Haemophilus influenza, typ e B meningitis, Haemophilus influenza, type B or HIB is it bacteria normally found in our nose and throat that can lead to very serious life threatening infections and no matter what I did in that day and into the night in terms of prompt antibiotics and she'd just been sick a few hours and, and fluids and all the, you know, ventilators and all the best things that modern medicine, she died, the vaccine for hip was not available until the 1990s. And until it did become available, hip disease affected approximately 25,000 children each year with things like meningitis, pneumonia, and bloodstream infections. And at that time in the hospital that I was practicing at any one time, there were generally five or six patients that had Haemophilus meningitis or invasive disease or some complication of this particular infection. And we knew that from the basic science that if you had antibody to the capsule or to the coat of the organism, that you were protected from disease. But we really didn't know how to make little kids make antibody. -

Ffontiau Cymraeg
This publication is available in other languages and formats on request. Mae'r cyhoeddiad hwn ar gael mewn ieithoedd a fformatau eraill ar gais. [email protected] www.caerphilly.gov.uk/equalities How to type Accented Characters This guidance document has been produced to provide practical help when typing letters or circulars, or when designing posters or flyers so that getting accents on various letters when typing is made easier. The guide should be used alongside the Council’s Guidance on Equalities in Designing and Printing. Please note this is for PCs only and will not work on Macs. Firstly, on your keyboard make sure the Num Lock is switched on, or the codes shown in this document won’t work (this button is found above the numeric keypad on the right of your keyboard). By pressing the ALT key (to the left of the space bar), holding it down and then entering a certain sequence of numbers on the numeric keypad, it's very easy to get almost any accented character you want. For example, to get the letter “ô”, press and hold the ALT key, type in the code 0 2 4 4, then release the ALT key. The number sequences shown from page 3 onwards work in most fonts in order to get an accent over “a, e, i, o, u”, the vowels in the English alphabet. In other languages, for example in French, the letter "c" can be accented and in Spanish, "n" can be accented too. Many other languages have accents on consonants as well as vowels. -

(3) (Y'(Uf)\L + 2A(U)B(Y(U))\\O
proceedings of the american mathematical society Volume 34, Number 1, July 1972 PROPERTIES OF SOLUTIONS OF DIFFERENTIAL EQUATIONS OF THE FORM / 4- a(t)b(y) = 0 ALLAN KROOPNICK Abstract. Two theorems are presented which guarantee the boundedness and oscillation of solutions of certain classes of second order nonlinear differential equations. In this paper we shall show boundedness of solutions of the differential equation y"+a(t)b(y)=0 with appropriate conditions on a(t) and b(y). Furthermore, all solutions will be oscillatory subject to a further condition. By oscillatory we shall mean a function having arbitrarily large zeros. The first theorem is an extension of a result of Utz [1] in which he con- siders b(y)=y2n~1. We now prove a result for boundedness of solutions. Theorem I. If a(t)> a>0 and da/dt^O on [T, oo), b(y) continuous, and limj,^±00B(y)=$yb(u) du=co then all solutions of (1) / + a(t)b(y)=0 are bounded as r—>-oo. Proof. Multiply (1) by 2/ to get (2) 2y'y" + 2a(t)b(y)y' = 0. Upon integration by parts we have (3) (y'(uf)\l+ 2a(u)B(y(u))\\o- 2 P df B(y)du = 0. J ir, Uli Thus (4) y'(tf + 2a(t)B(y(t))- 2 f ^ B(y)du = K Jto du where K=y'(t0)2-r-2a(to)B(y(to)). Now the above implies \y'\ and \y\ remain bounded. If not, the left side of (4) would become infinite which is impossible. -

Vaccine Hesitancy
Vaccine Hesitancy Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g. -

У^C/ ^Xaxxlty^Yy Yyy- Y/A М /A XX
1 5 2 Va X A y // /^ //X A ^ M a i Y'YY/ ’^y y y -ì - A Y & y n / X X - y7 AAl/XX /AlAX AA /X y ^z^s -i o y ^ y y H ^ - z ^ A c ^ J Ì^Ystn^XUY Y LY X ’YYi yl^-CY7 A/ y A A Y ^ t V Y f AC-, C x ix x r~/Y- CYtS Vy* X{ l à / ¿ Y l ^ l Y A". A X r u / , A / C A ut Y A l' T O tl / V l X ^ ì y y Z A ^/Aa ^ A y y A c y ^ yl A A t d l f H ^ c ò / a AtA r t / ^ ¿ ù a / z Cy/ X X-CAX? yCA %-O > yCCfl/Cj t L ^ H ' Ì ^ ì y AC A & A / c i Cy y ^ ehf yZy y y Y- ACC y i C C A A ^ tiY 'l'VZY-YcZo 'SA y y - Z-eY^Y A iaA a ^ C c' A^ . 7 ' Y ^ C / / ¿ H y/yi-C-td A#A y -AA Yy y y A 'iCA uX ZY lls X^^y y y y y y y A y '* 1 Xt i 'l-tA Y V Y l' '/A A in - r iY -t^Y d X y v A y y > y Y Y Y y CitYYyACAty C/ y y y 'lA 'V ¿A,A a 4 aC y y A i Y Y y 'Y> '-/AYYA •■*yv/ ’a a a i a U ^ t & l i - AA-Y>t/ACy-7A ^ < / A - '" 'X X X 'X t X d'PY'. -

1. First Examples a Differential Equation for a Variable U(X, Y, . . .)
1. First examples A differential equation for a variable u(x; y; : : : ), which depends on the independent variables x, y, . , is an equation which involves u and some of its partial derivatives. Differential equations have traditionally played a huge role in the sciences and so they have been heavily studied in mathematics. If u depends on x and y, there are two partial derivatives, @u @u and : @x @y Since partial differential equations might involve lots of variables and derivatives, it is convenient to introduce some more compact notation. @u @u = u and = u : @x x @y y Note that @2u @2u @2u u = u = and u = : xx @x2 xy @x@y yy @y2 Recall some basic facts about derivatives. First of all derivatives are local, meaning if you only change the function away from a point then the derivative is unchanged (contrast this with the integral, which is far from local; the area under the graph depends on the global behaviour). Secondly if all 2nd derivatives exist and they are continuous then the mixed partials are equal: uxy = uyx: Example 1.1. Suppose that u(x; y) = sin(xy): Then ux = y cos(xy) and uy = x cos(xy): But then uyx = cos(xy) − xy sin(xy) and uxy = cos(xy) − xy sin(xy): Partial differential equations are in general very complicated and normally we can only make sense of the solutions of those equations which come from applications. If there are two space variables then we typically call them x and y but as usual t denotes a time variable and x a space variable. -

The Challenge of Achieving Universal Access to Vaccines
Keynote Address: The Challenge of Achieving Universal Access to Vaccines WIPO & AMF Seth Berkley M.D, CEO 8 November 2017 www.gavi.org 1 Vaccine landscape WIPO 8 November 2017 The world before vaccines Examples of major disease outbreaks Polio Cholera New York pandemic 1916 Europe 6,000 deaths 1829-1851 >200,000 deaths Yellow fever Philadelphia Smallpox 1793 epidemic >5,000 India deaths 1974 15,000 deaths Flu pandemic 1918-1920 > 50-100 million deaths worldwide WIPO 8 November 2017 History of vaccine development Source: Delaney et.al. 2014 WIPO 8 November 2017 Cumulative number of vaccines developed licensed 50+ vaccines Lyme OspA, protein (1998) Tuberculosis (Bacille Calmette-Guérin) Pneumococcal conjugate, heptavalent* (2000) 1927 Typhus Varicella (1995) Meningococcal quadrivalent conjugates* (2005) Japanese encephalitis (Vera-cell) (2009) 1938 Cholera (recombinant Toxin B) # (1993) Cholera (WC only) (2009) Tetanus toxoid Cholera (WC-rBC) (1991) Human papillomavirus recombinant bivalent (2009) Influenza H. influenzaeconjugate* (1987) Quadrivalent influenza vaccines (2012) Typhoid Cholera Pertussis 1936 Meningococcal C and Y and 1886 1896 1926 H. influenza type B polysaccharide (1985) Haemophilusinfluenza type b (2012) Adenovirus (1980) Meningococcal B (2013) Smallpox Diphtheria Yellow Rabies, cell culture (1980) Influenza vaccine (baculovirus) (2013) 1798 toxoid Fever Typhoid conjugate vaccine (2013) Rabies Plague Meningococcal polysaccharides (1974) 1923 1935 Human papillomavirus, 9-valent (2014) 1885 Rubella, live (1969) 1897 vaccines