Monitoring Cellular Movement in Vivo with Photoconvertible Fluorescence Protein ‘‘Kaede’’ Transgenic Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Microlymphatic Surgery for the Treatment of Iatrogenic Lymphedema
Microlymphatic Surgery for the Treatment of Iatrogenic Lymphedema Corinne Becker, MDa, Julie V. Vasile, MDb,*, Joshua L. Levine, MDb, Bernardo N. Batista, MDa, Rebecca M. Studinger, MDb, Constance M. Chen, MDb, Marc Riquet, MDc KEYWORDS Lymphedema Treatment Autologous lymph node transplantation (ALNT) Microsurgical vascularized lymph node transfer Iatrogenic Secondary Brachial plexus neuropathy Infection KEY POINTS Autologous lymph node transplant or microsurgical vascularized lymph node transfer (ALNT) is a surgical treatment option for lymphedema, which brings vascularized, VEGF-C producing tissue into the previously operated field to promote lymphangiogenesis and bridge the distal obstructed lymphatic system with the proximal lymphatic system. Additionally, lymph nodes with important immunologic function are brought into the fibrotic and damaged tissue. ALNT can cure lymphedema, reduce the risk of infection and cellulitis, and improve brachial plexus neuropathies. ALNT can also be combined with breast reconstruction flaps to be an elegant treatment for a breast cancer patient. OVERVIEW: NATURE OF THE PROBLEM Clinically, patients develop firm subcutaneous tissue, progressing to overgrowth and fibrosis. Lymphedema is a result of disruption to the Lymphedema is a common chronic and progres- lymphatic transport system, leading to accumula- sive condition that can occur after cancer treat- tion of protein-rich lymph fluid in the interstitial ment. The reported incidence of lymphedema space. The accumulation of edematous fluid mani- varies because of varying methods of assess- fests as soft and pitting edema seen in early ment,1–3 the long follow-up required for diagnosing lymphedema. Progression to nonpitting and irre- lymphedema, and the lack of patient education versible enlargement of the extremity is thought regarding lymphedema.4 In one 20-year follow-up to be the result of 2 mechanisms: of patients with breast cancer treated with mastec- 1. -

M. H. RATZLAFF: the Superficial Lymphatic System of the Cat 151
M. H. RATZLAFF: The Superficial Lymphatic System of the Cat 151 Summary Four examples of severe chylous lymph effusions into serous cavities are reported. In each case there was an associated lymphocytopenia. This resembled and confirmed the findings noted in experimental lymph drainage from cannulated thoracic ducts in which the subject invariably devdops lymphocytopenia as the lymph is permitted to drain. Each of these patients had com munications between the lymph structures and the serous cavities. In two instances actual leakage of the lymphography contrrult material was demonstrated. The performance of repeated thoracenteses and paracenteses in the presenc~ of communications between the lymph structures and serous cavities added to the effect of converting the. situation to one similar to thoracic duct drainage .The progressive immaturity of the lymphocytes which was noted in two patients lead to the problem of differentiating them from malignant cells. The explanation lay in the known progressive immaturity of lymphocytes which appear when lymph drainage persists. Thankful acknowledgement is made for permission to study patients from the services of Drs. H. J. Carroll, ]. Croco, and H. Sporn. The graphs were prepared in the Department of Medical Illustration and Photography, Dowristate Medical Center, Mr. Saturnino Viloapaz, illustrator. References I Beebe, D. S., C. A. Hubay, L. Persky: Thoracic duct 4 Iverson, ]. G.: Phytohemagglutinin rcspon•e of re urctcral shunt: A method for dccrcasingi circulating circulating and nonrecirculating rat lymphocytes. Exp. lymphocytes. Surg. Forum 18 (1967), 541-543 Cell Res. 56 (1969), 219-223 2 Gesner, B. M., J. L. Gowans: The output of lympho 5 Tilney, N. -

Patterns of Lymphatic Drainage in the Adultlaboratory
J. Anat. (1971), 109, 3, pp. 369-383 369 With 11 figures Printed in Great Britain Patterns of lymphatic drainage in the adult laboratory rat NICHOLAS L. TILNEY Department of Surgery, Peter Bent Brigham Hospital, and Harvard Medical School, Boston, Massachusetts (Accepted 27 April 1971) INTRODUCTION This study was undertaken to define and elucidate patterns of lymphatic drainage in the adult laboratory rat. The incentive for the work arose from investigations into the role of regional lymphatics in the sensitization of the host by skin allografts. It has become clear that the response of rats to antigens, investigated increasingly in the available inbred strains, requires an accurate knowledge of lymphoid anatomy and lymphatic drainage routes. Examinations of the lymphatics of specific body areas of the rat have appeared sporadically in the literature, but descriptions of regional drainage patterns, especially of peripheral sites, are unavailable. Previous investigations by Job (1919), Greene (1935) and Sanders & Florey (1940) have con- centrated primarily upon the location of the lymphoid tissues. Miotti (1965) has stressed visceral drainage, and Higgins (1925) has described the lymphatic system of the newborn rat. A more complete definition of both somatic and visceral lymphatic routes is presented. MATERIALS AND METHODS One hundred and thirty normal adult rats of both sexes, each weighing between 150 and 300 g, were studied. The animals came from five strains: each inbred - Oxford strains of the albino (AO), hooded (HO), agouti (DA), and F1 hybrid of the HO and DA strains - and 'stock' animals from a closed outbred albino colony. Under ether anaesthesia, the site for cutaneous injection was clipped or a serous cavity entered for visceral injection. -

Extension of Cervical Cancer to the Superficial Inguinal Lymph Nodes
5 Junie 1965 S.A. TYDSKRIF VIR OBSTETRIE E GINEKOLOGIE 23 assessed and controlled. Adequate control in patients with REFERENCES 1. Kirk. H. H. (1958): J. Obstet. Gynaec. Brit. Emp.. 6S. 387. Addison's disease appears (a) to enhance the fertility, (b) 2. Fitzpatrick. K. G. (1922): Surg. Gynec. Obstet., 35, 72. to reduce the risk at the time of delivery and (c) to im 3. Brent. F. (1950): Amer. J. Surg.. 79. 645. 4. Rolland. C. F .. Matthews. J. D. and Matthew. G. D. (1953): J. prove the prospects of successful breast feeding. Obstet. Gynaec. Brit. Emp.. 60. 57. 5. Francis. H. H. and Forster. J. C. (1958): Proc. Roy. Soc. Med.. SI. 513. SUMMARY 6. Browne. F. J. and Browne. J. C. McClure (1963): Briris" Obstetric Practice, 3rd ed.. p. 484. London: Heinemann. 7. O·Sullivan. D. (1954): J. Irish Med. Assoc.. 36, 315. A case is presented of a 26-year-old patient known to have 8. Sluder. H. M. (1959): Amer. J. Obstet. Gynec.. 78, 808. Addison's disease who became pregnant. 9. Allahbadia. N. K. (1960): J. Obstet. Gynaec. Brit. Emp.. 67, 641. 10. Papper. E. M. and Cahill. G. F. (1952): J. Amer. Med. Assoc., Careful antenatal and intrapartum supervision by both 148. 174. 11. Jailer. J. W. and Knowlton. A. I. (1950): J. Clin. Invest.. 29, 1430. physician and obstetrician ensures that the patient has the 12. Moore. F. H. and Freedman. J. R. (1956): Amer. J. Obstet. Gynec.. best chance of a successful outcome. 72. 1340. 13. Gabrilove. J. L. and Schval. -

Spontaneous Infarction of Superficial Lymph Nodes
J Clin Pathol: first published as 10.1136/jcp.25.8.689 on 1 August 1972. Downloaded from J. clin. Path., 1972, 25, 689-696 Spontaneous infarction of superficial lymph nodes J. DOUGLAS DAVIES AND A. G. STANSFELD' From the Department of Histopathology, St Bartholomew's Hospital, London SYNOPSIS Five cases of extensive infarction of lymph nodes were traced in just over 16 years' surgical material. All presented with painful swelling in a superficial lymph node chain. None was diagnosed clinically; two were interpreted as fibroadenoma of the axillary tail of the breast, and two as a femoral hernia. Microscopically the lymph nodes in the first three weeks after infarction were characterized by extensive necrosis of medullary and cortical lymphoid cells, but the central reticulin architecture and a narrow, incomplete rim of viable subcapsular lymphoid tissue were preserved. Reactive perinodal inflammation and the formation of granulation tissue resembled the reaction to myocardial infarction. The late stage of the lesion was characterized by incomplete regeneration of lymphoid tissue in the lymph nodes. The lesions appeared attributable to thrombosis of veins within the substance and the hila of the nodes. Though experimental infarction of lymph nodes has were stained by Harris' haematoxylin and eosin, and been produced in dogs (Holman and Self, 1938; Gordon and Sweet's method for reticulin. When Tilak and Howard, 1964; Kister, Conklin, and appropriate PAS, Gram, or Ziehl-Neelsen prepara- copyright. Habif, 1965) and rabbits (Osogoe and Courtice, tions were examined. 1968), spontaneous infarction of previously normal lymph nodes in man has rarely been recorded Case Reports (Holman and Self, 1938). -

Analysis of Lymphatic Drainage in Various Forms of Leg Edema Using Two Compartment Lymphoscintigraphy
43 Lymphology 31 (1998) 43-55 ANALYSIS OF LYMPHATIC DRAINAGE IN VARIOUS FORMS OF LEG EDEMA USING TWO COMPARTMENT LYMPHOSCINTIGRAPHY P. Brautigam, E. FOldi, I. Schaiper, T. Krause, W. Vanscheidt, E. Moser Abteilung Nuklearmedizin, Radiol. University Klinik Freiburg (PB,TK,EM), FOldiklinik, Fachklinik ftir lymphologische Erkrankungen, Hinterzarten (EF), Zentrum ftir Nuklearmedizin, Paracelsus- Klinik Osnabrtick (IS), and Universitats-Hautklinik, Freiburg (WV), Germany ABSTRACT was reduced. Patients with lipedema (obesity) scintigraphically showed no alteration in The anatomical and functional status lymphatic transport. of the epifascial and subfascial lymphatic This study demonstrates that lymphatic compartments was analyzed using two drainage is notably affected (except in obesity compartment lymphoscintigraphy in five termed lipedema) in various edemas of the leg. groups of patients (total 55) with various Lymphatic drainage varied depending on the forms of edema of the lower extremities. specific compartment and the pathophysiologic Digital whole body scintigraphy enabled mechanism accounting for the edema. Two semiquantitative estimation of radio tracer compartment lymphoscintigraphy is a valuable transport with comparison of lymphatic diagnostic tool for accurate assessment of leg drainage between those individuals without edema of known and unknown origin. (normal) and those with leg edema by calculating the uptake of the radiopharma- The lymphatic system is deranged in ceutical transported to regional lymph nodes. various forms of leg edema of both lymphatic A visual assessment of the lymphatic drainage and non-lymphatic origin including venous- pathways of the legs was also performed. lymphedema, cyclic idiopathic edema, and In patients with cyclic idiopathic edema, lipedema (obesity) (1,2). As no discrete an accelerated rate of lymphatic transport differences exist on clinical grounds among was detected (high lymph volume overload or the various edema syndromes, differential dynamic insufficiency). -

Lymph Flow in Human Leg Kiyoshi Seki
Lymphology 12 (I 979) 2- 3 Lymph Flow in Human Leg Kiyoshi Seki 3rd Dept. Internal Medicine, Toho University, Tokyo, Japan Summary Clearance rate of subcutaneously injected A little bit of RJSA (0.01 - 0.02 ml) was injected into RJSA was rapid in cardiac, nephrotic and he a lymphatic at t11e back of a human foot in supine patic edema and slow in lymphedema (2, 3). and/or sitting position, and U1e radioactivity curves From this it was assumed that lymph flow were obtained at the inguinal and/or at the middle of U1e thigh. Radioactivity curves at the inguinal region was rapid or increased in cardiac edema as in showed stepwise rise with the frequency of about nephrotic and hepatic edema. I /min. in subjects without edema and steep rise without staircase in patients with cardiac edema. ln tissue clearance of RISA, the subjects are Radioactivity curves at the middle of the thigh showed hospitalized and kept in bed as quiet as many spiky waves with the frequency of about 1/min. possible. However, as the observations are in supine subjects without edema, which were increased carried out 2 to 3 days long, effect of the in frequency, duration and/or height in sitting subjects without edema and also in supine and sitting patients movement of the subjects on the lymph flow with cardiac edema. may not be neglibly small. Therefore, it may Therefore, it may be said that there are rhythmic lymph be difficult to compare the results by dye Oows in lower leg of subjects without edema and of injection method with those by tissue clearan patients with cardiac edema not only in supine but ce of RJSA. -

Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer
Journal of Clinical Medicine Review Utility of Minimally Invasive Technology for Inguinal Lymph Node Dissection in Penile Cancer Reza Nabavizadeh 1,* , Benjamin Petrinec 1, Andrea Necchi 2, Igor Tsaur 3, Maarten Albersen 4 and Viraj Master 1 1 Department of Urology, Emory University School of Medicine, Atlanta, GA 30322, USA; [email protected] (B.P.); [email protected] (V.M.) 2 Department of Medical Oncology, Fondazione IRCCS Istituto Nazionale dei Tumori, 20133 Milan, Italy; [email protected] 3 Department of Urology and Pediatric Urology, University Medicine Mainz, 55131 Mainz, Germany; [email protected] 4 Department of Urology, University Hospitals Leuven, 3000 Leuven, Belgium; [email protected] * Correspondence: [email protected]; Tel.: +1-310-986-0966; Fax: +1-404-778-4231 Received: 9 June 2020; Accepted: 30 July 2020; Published: 3 August 2020 Abstract: Our aim is to review the benefits as well as techniques, surgical outcomes, and complications of minimally invasive inguinal lymph node dissection (ILND) for penile cancer. The PubMed, Wiley Online Library, and Science Direct databases were reviewed in March 2020 for relevant studies limited to those published in English and within 2000–2020. Thirty-one articles describing minimally invasive ILND were identified for review. ILND has an important role in both staging and treatment of penile cancer. Minimally invasive technologies have been utilized to perform ILND in penile cancer patients with non-palpable inguinal lymph nodes and intermediate to high-risk primary tumors or patients with unilateral palpable non-fixed inguinal lymph nodes measuring less than 4 cm, including videoscopic endoscopic inguinal lymphadenectomy (VEIL) and robotic videoscopic endoscopic inguinal lymphadenectomy (RVEIL). -

Adenocarcinoma of the Lung with Inguinal Lymph Node Metastasis
Open Access Case Report DOI: 10.7759/cureus.13658 Adenocarcinoma of the Lung With Inguinal Lymph Node Metastasis Venkatkiran Kanchustambham 1 , Swetha Saladi 2 1. Pulmonary and Critical Care Medicine, University of North Dakota, Fargo, USA 2. Internal Medicine, University of North Dakota School of Medicine and Health Sciences, Grand Forks, USA Corresponding author: Swetha Saladi, [email protected] Abstract Adenocarcinoma of the lung can present with distant metastasis, with major metastasis sites being mediastinal lymph nodes, liver, brain, and adrenal glands. Inguinal lymph nodes are an unusual site for distant metastasis of adenocarcinoma of the lung. We discuss the case of a 73-year-old Caucasian female with a medical history significant for hypertension, chronic obstructive pulmonary disease (COPD) who was seen in the primary care clinic for ongoing shortness of breath, worsening cough, and wheezing. She was prescribed a short course of steroids and antibiotics for possible COPD exacerbation. Despite these measures, the patient had worsening pulmonary symptoms and got evaluated in the emergency room. A CT scan of the chest showed right upper lobe bilobed masses and bulky mediastinal lymph nodes resulting in the partial collapse of the lung's right upper lobe. She got admitted to the hospital for further evaluation, and pulmonary service was consulted for possible endobronchial ultrasound-guided biopsy (EBUS) of the mediastinal nodes. During the physical exam, she was found to have a large fungating mass in the right groin. Upon further questioning, she reported that the mass began as a small swelling in the groin three months ago and was evaluated by the primary care physician and received antibiotics for two weeks. -

A Case of Inguinal Lymph Node Squamous Cell Carcinoma of Unknown Origin, Accompanied with Carcinoma in Situ of Cervix
J Gynecol Oncol Vol. 19, No. 2:145-149, June 2008 DOI:10.3802/jgo.2008.19.2.145 Case Report A case of inguinal lymph node squamous cell carcinoma of unknown origin, accompanied with carcinoma in situ of cervix Sung-Ha Lee1, Min-Jung Kim1, Hee-Jung Lee2, Sa-Jin Kim1, Jong-Sup Park1, Soo-Young Hur1 Departments of 1Obstetrics and Gynecology, 2Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea Metastatic Cancer of Unknown Primary Site (CUP) accounts for approximately 3-5% of all malignant neoplasms. CUP represents a heterogeneous group of metastatic tumors for which no primary site can be detected following a thorough medical history, careful clinical examination, and extensive diagnostic work-up. Several authors have reported poor prognosis of this malignancy, because there is no consensus on diagnostic guidelines and optimal therapy. Historically, chemotherapy has been the cornerstone of treatment for patients with CUP. We experienced a case of inguinal lymph node squamous cell carcinoma of unknown origin, accompanied with carcinoma in situ of the cervix. We report this case with a brief review of the literatures. Key Words: Metastatic cancer of unknown primary site, Inguinal lymph node INTRODUCTION origin, and the primary lesion could be found in only 35 patients (55%). As the primary site, the lungs, the pancreas Metastatic cancer of unknown primary site (CUP) accounts and the bile duct system, and the gastro-intestinal system for 3-5% of all malignant neoplasms, and it is defined as were most prevalent. It was more difficult to find the primary metastatic cancer from an unknown primary site, for which no lesion in poorly differentiated carcinoma cases.4 Recently, original site can be detected even after performing all possible upon reporting the role of positron emission tomography tests.1,2 In the 1970s, some researchers argued that the (PET) scan in various fields, the diagnostic technique to find diagnosis of CUP could be made only if the primary site could the unknown primary site has become became advanced. -

Antigenin Breastcancer
CLINICAL SCIENCES INVEST1GA11VE NUCLEAR MEDICINE Axillary Lymphoscintigraphy by Radioimmunodetection of Carcinoembryonic Antigenin BreastCancer Frank H. DeLand,E. EdmundKim, Robert L. Corgan, Scott Casper, F. James Primus, Ellen Spremulli, Norman Estes, and DavidM.Goldenberg Universityof KentuckyCollegeof Medicine,VeteransAdministrationMedicalCenter,andEphraimMcDowell CommunityCancerNetwork,Inc.,Lexington,Kentucky In seven women with carcinoma of the breast 1-131-labeled antIbodies to card noembryonic antigen (CEA) were administered subcutaneously in the finger webs. Subsequent scintlgraphic images demonstrated localization of radioactivity in the ipsilateral axlllary metastases of all patients and in the contralateral axillae of three. Fifteen patients wfth either gastrointestinal or genitourinary cancers were studied as controls; in 12 both the hands and feet were injected wfth antibodies to CEA and in the other three either the hands or feet. Radioactivity was observed in theinquinalnodesoffourcontrolpatientswithtumorsbelowthediaphragmandin the axillary nodes of one patient with a tumor above the diaphragm. The concen tration of antibody in lymph node metastases from breast carcinoma was 100% specific. In those lymph nodes that presumably contained no metastatic tumor but demonstrated localization of labeled antibody, retention of CEA in the nodes from tumor drainage is postulated as the receptor site for the antibody. J NucI Med 20 1243-1250, 1979 More than 25 years ago Sherman et al. (I ) demon specific deposition in lymph nodes. We now report the strated regional lymph nodes by external scintillation USC of a radionuclide-labeled antibody to a tumor-asso imaging following the interstitial administration of ciatcd antigen, carcinoembryonic antigen (CEA), for the colloidal radioactive gold. Subsequently, other labeled scintigraphic detection of axillary lymph-node metas colloids have been used for lymphoscintigraphy. -
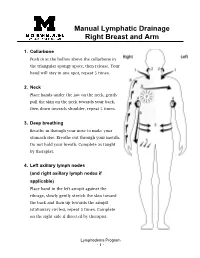
Manual Lymphatic Drainage Right Breast and Arm
Manual Lymphatic Drainage Right Breast and Arm 1. Collarbone Push in at the hollow above the collarbone in the triangular spongy space, then release. Your hand will stay in one spot, repeat 5 times. 2. Neck Place hands under the jaw on the neck, gently pull the skin on the neck towards your back, then down towards shoulder, repeat 5 times. 3. Deep breathing Breathe in through your nose to make your stomach rise. Breathe out through your mouth. Do not hold your breath. Complete as taught by therapist. 4. Left axillary lymph nodes (and right axillary lymph nodes if applicable) Place hand in the left armpit against the ribcage, slowly gently stretch the skin toward the back and then up towards the armpit (stationary circles), repeat 5 times. Complete on the right side if directed by therapist. Lymphedema Program - 1 - 5. Right inguinal lymph nodes Place hands just below hip crease, gently pull skin in and then up (stationary circles), repeat 5 times. 6. Sweeping Lightly “sweep” from right axillary lymph nodes to right inguinal lymph nodes (from right armpit to the right top of thigh). Then lightly “sweep” across the chest from right axillary lymph nodes to Left axillary lymph nodes (from right armpit to left arm pit). Repeat both actions 5 times. 7. Breast massage • With a light touch, do small circles starting at top of breast going in a spiral motion always gently pulling outward until you hit the nipple. 2-3 times. • Pull from the nipple out towards your chest in short light strokes. 2-3 times.