Chapter 3 Nutrient Origins and Pathways in the Manning Valley
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scenic Drives Gloucester New South Wales DRIVE 2: GLOUCESTER TOPS
Enter this URL to view the map on your mobile device: https://goo.gl/maps/c7niuMo3oTr Scenic Drives Gloucester New South Wales DRIVE 2: GLOUCESTER TOPS Scenic Drive #2: Gloucester Tops return via Faulkland Length: 115km Start: Visitor Information Centre at 27 Denison Street End: Gloucester township Featuring: Gloucester River valley, Gloucester Tops, Barrington Tops National Park, Andrew Laurie Lookout, Gloucester Falls, Gloucester River, Faulkland and multiple river crossings on concrete causeways (caution advised). Gloucester Visitor Information Centre 27 Denison Street, Gloucester New South Wales AUSTRALIA T: 02 6538 5252 F: 02 6558 9808 [email protected] www.gloucestertourism.com.au Scenic Drive #2 – Gloucester Tops And on the way you’ll see beautiful rural landscapes and The Antarctic Beech Forest Track features cool tem- cross numerous river fords with picnic and swimming perate rainforest with the canopy of ancient trees If you only have half a day then this offers you a taste spots before returning to Gloucester or continuing your towering above the tree ferns and a damp carpet of of world heritage wilderness. Gloucester Tops Nation- journey towards the Pacific Highway. moss on the forest floor, rocks and logs. The longer al Park is the easternmost section of Barrington Tops walking track option takes you to a mossy cascade and is the closest part of this stunning wilderness to Along Gloucester Tops Road for the next 40km you’ll with the purest mountain water. As you step be- Gloucester. track the Gloucester River as the road winds through hind the curtain of green you’ll feel like you’re on productive farming valleys surrounded by forest-clad the film set of Lord Of The Rings. -

Water Quality in the Manning River Estuary Is Made by Determining to What Extend These Long-Term Goals Were Being Met
I ~ I i.; I ! I I Ii Ii GRE~~TER Ti\REE I II II:1 d CITY COlJNCIL !I I :! I I -'" ... ,,... .. , ... I I I I I I I- I I WATER QUAllIT IN THE MANNING RIVER . I 1989 - 94 I I 1- I I- I I I I I -I ~_ ... ~ __•. .(-."._.,~ .............. -.--=r ,.---' _. I I I I I I I I~ a report prepared by I Anna Kaliska I SEWER & WASTE SERVICES CRE_ATER TAREE CITY COUNCIL 2 Palteney Street I: TAREE NSW 2430 . Phone (06S) 913 399 I, October 1994 I !::", I I I I I I 1 INTRODUCTION I 2 MONITORING PROGRAM I 2.1 Stations Location 2.2 Flow Conditions During Sample Collection '1 ····2.3 Parameters-Neasl:<.reci·- . -- .,. .... .......-;:;..:;._._... --. -:::;;....: .. -' I 3 TUE MANNING RIVER SYSTEM 3.1 Catchment Description I 3.2 Tidal Behaviour and Sediment Transport 3.3 Water Resources and the River Flow I 4 TUE SOURCES OF WATER POLLUTION I 4.1 Point Source Discharges 4.2 Diffuse Source Pollution I I 5 WATER QUALITY IN TUE MANNING RIVER ESTUARY 5.1 Salinity 5.2 Dissolved Oxygen I 5.3 Biochemical Oxygen Demand 5.4 Clarity 5.5 Bacteriological Characteristics I 5.6 Nutrients I 6 CONCLUSIONS I REFERENCES I I 'I I TABLES 1 1 Water Quality Sampling Stations 2 Manning River Flow at Killawarra Station I 3 Estimated Manning River Flow at Taree 1 4 Characteristics of Sewage Treatment Plant Effluent I FIGURES I I 1 Location of Sample Collection Sites 2 Tidal Characteristics of the Manning River Estuary I 3 Load Discharged to the Manning River Before and after Taree Sewage Treatment Plant Flow Diversion I 4 Salinity - Mean Values 1989-94 1 5 Surface Dissolved Oxygen 1989-94 6 Bottom Dissolved Oxygen 1989-94 I 7 Average Dissolved Oxygen Ratios 1986, I 91/92 and 93/94 8 Biochemical Oxygen Demand 1989-94 I 9 Secchi Disc 1989-94 I 10 Faecal Coliforms 1989-94 11 Faecal Coliform 1989/92 and 93/94 I 12 Total Phosphorus 1989-94 ,-'Jo I, 1,3 Total Nitrogen 1989-94 14 Total Phosphorus 1984-94 15 Total Nitrogen 1984-94 = I I 1 1 INTRODUCTION I The Manning River system is one of the major river systems in New South Wales and is seen as an important natural resource on a local, regional and state level. -

The Formation of the Half-Time Schools of New South Wales
“Every locality, however remote, and every family, however humble”: the formation of the Half-time schools of New South Wales 1866-1869 A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy at Charles Sturt University by Ashley Thomas Freeman DipT (Armidale CAE), BEd (Canberra CAE), MEd (UNE) Charles Sturt University August 2009 Table of Contents Certificate of authorship .................................................................................. i Acknowledgements ....................................................................................... iii Abstract .......................................................................................................... v Chapter One Introduction ............................................................................... 1 The Half-time schools of New South Wales .............................................. 5 Objective of this thesis ............................................................................... 8 Significance of this thesis ........................................................................... 8 Principal questions ................................................................................... 10 Methodology ............................................................................................ 11 Writer‟s position ....................................................................................... 18 Sources and literature reviewed ............................................................... 19 Context -
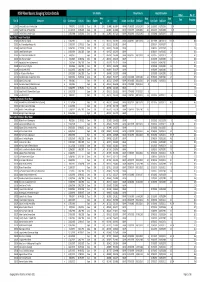
Gauging Station Index
Site Details Flow/Volume Height/Elevation NSW River Basins: Gauging Station Details Other No. of Area Data Data Site ID Sitename Cat Commence Ceased Status Owner Lat Long Datum Start Date End Date Start Date End Date Data Gaugings (km2) (Years) (Years) 1102001 Homestead Creek at Fowlers Gap C 7/08/1972 31/05/2003 Closed DWR 19.9 -31.0848 141.6974 GDA94 07/08/1972 16/12/1995 23.4 01/01/1972 01/01/1996 24 Rn 1102002 Frieslich Creek at Frieslich Dam C 21/10/1976 31/05/2003 Closed DWR 8 -31.0660 141.6690 GDA94 19/03/1977 31/05/2003 26.2 01/01/1977 01/01/2004 27 Rn 1102003 Fowlers Creek at Fowlers Gap C 13/05/1980 31/05/2003 Closed DWR 384 -31.0856 141.7131 GDA94 28/02/1992 07/12/1992 0.8 01/05/1980 01/01/1993 12.7 Basin 201: Tweed River Basin 201001 Oxley River at Eungella A 21/05/1947 Open DWR 213 -28.3537 153.2931 GDA94 03/03/1957 08/11/2010 53.7 30/12/1899 08/11/2010 110.9 Rn 388 201002 Rous River at Boat Harbour No.1 C 27/05/1947 31/07/1957 Closed DWR 124 -28.3151 153.3511 GDA94 01/05/1947 01/04/1957 9.9 48 201003 Tweed River at Braeside C 20/08/1951 31/12/1968 Closed DWR 298 -28.3960 153.3369 GDA94 01/08/1951 01/01/1969 17.4 126 201004 Tweed River at Kunghur C 14/05/1954 2/06/1982 Closed DWR 49 -28.4702 153.2547 GDA94 01/08/1954 01/07/1982 27.9 196 201005 Rous River at Boat Harbour No.3 A 3/04/1957 Open DWR 111 -28.3096 153.3360 GDA94 03/04/1957 08/11/2010 53.6 01/01/1957 01/01/2010 53 261 201006 Oxley River at Tyalgum C 5/05/1969 12/08/1982 Closed DWR 153 -28.3526 153.2245 GDA94 01/06/1969 01/09/1982 13.3 108 201007 Hopping Dick Creek -

'Geo-Log' 2016
‘Geo-Log’ 2016 Journal of the Amateur Geological Society of the Hunter Valley Inc. Contents: President’s Introduction 2 Gloucester Tops 3 Archaeology at the Rocks 6 Astronomy Night 8 Woko National Park 11 Bar Beach Geology and the Anzac Walkway 15 Crabs Beach Swansea Heads 18 Caves and Tunnels 24 What Rock is That? 28 The Third Great Numbat Mystery Reconnaissance Tour 29 Wallabi Point and Lower Manning River Valley Geology 32 Geological Safari, 2016 36 Social Activities 72 Geo-Log 2016 - Page 1 President’s Introduction. Hello members and friends. I am pleased and privileged to have been elected president of AGSHV Inc. for 2016. This is an exciting challenge to be chosen for this role. Hopefully I have followed on from where Brian has left off as he has left big shoes to fill. Brian and Leonie decided to relinquish their long held posts as President and Treasurer (respectively) after many years of unquestionable service to our society, which might I say, was carried out with great efficiency and grace. They have set a high standard. Thank you Brian and Leonie. We also welcomed a new Vice President, Richard Bale and new Treasurer John Hyslop. Although change has come to the executive committee the drive for excellence has not been diminished. Brian is still very involved with organising and running activities as if nothing has changed. The “What Rock Is That” teaching day Brian and Ron conducted (which ended up running over 2 days) at Brian’s home was an outstanding success. Everyone had samples of rocks, with Brian and Ron explaining the processes involved in how these rocks would have formed, and how to identify each sample, along with copious written notes and diagrams. -

Orocline-Driven Transtensional Basins: Insights from the Lower Permian Manning Basin
PUBLICATIONS Tectonics RESEARCH ARTICLE Orocline-driven transtensional basins: Insights 10.1002/2015TC004021 from the Lower Permian Manning Key Points: Basin (eastern Australia) • Structures in the Manning Basin confirm the existence of the Llyam White1, Gideon Rosenbaum1, Charlotte M. Allen2, and Uri Shaanan1 Manning Orocline • The Manning Basin is an Early 1School of Earth Sciences, University of Queensland, Brisbane, Queensland, Australia, 2Institute for Future Environments, Permian sinistral transtensional pull-apart basin Queensland University of Technology, Brisbane, Queensland, Australia • Spatiotemporal relationships link basin formation to oroclinal bending Abstract The New England Orogen in eastern Australia exhibits an oroclinal structure, but its geometry and geodynamic evolution are controversial. Here we present new data from the southernmost part of the Supporting Information: • Supporting Information S1 oroclinal structure, the Manning Orocline, which supposedly developed in the Early Permian, contemporaneously and/or shortly after the deposition of the Lower Permian Manning Basin. New U-Pb detrital zircon data provide Correspondence to: a maximum depositional age of ~288 Ma. Structural evidence from rocks of the Manning Basin indicates that L. White, both bedding and preoroclinal fold axial planes are approximately oriented parallel to the trace of the Manning [email protected] Orocline. Brittle deformation was dominated by sinistral strike-slip faulting, particularly along a major fault zone (Peel-Manning Fault System), which is marked by the occurrence of a serpentinitic mélange, and separates Citation: tectonostratigraphic units of the New England Orogen. Our revised geological map shows that the Manning White, L., G. Rosenbaum, C. M. Allen, and U. Shaanan (2016), Orocline-driven Basin is bounded by faults and serpentinites, thus indicating that basin formation was intimately linked to transtensional basins: Insights from the deformation along the Peel-Manning Fault System. -

Government Gazette of the STATE of NEW SOUTH WALES Number 112 Monday, 3 September 2007 Published Under Authority by Government Advertising
6835 Government Gazette OF THE STATE OF NEW SOUTH WALES Number 112 Monday, 3 September 2007 Published under authority by Government Advertising SPECIAL SUPPLEMENT EXOTIC DISEASES OF ANIMALS ACT 1991 ORDER - Section 15 Declaration of Restricted Areas – Hunter Valley and Tamworth I, IAN JAMES ROTH, Deputy Chief Veterinary Offi cer, with the powers the Minister has delegated to me under section 67 of the Exotic Diseases of Animals Act 1991 (“the Act”) and pursuant to section 15 of the Act: 1. revoke each of the orders declared under section 15 of the Act that are listed in Schedule 1 below (“the Orders”); 2. declare the area specifi ed in Schedule 2 to be a restricted area; and 3. declare that the classes of animals, animal products, fodder, fi ttings or vehicles to which this order applies are those described in Schedule 3. SCHEDULE 1 Title of Order Date of Order Declaration of Restricted Area – Moonbi 27 August 2007 Declaration of Restricted Area – Woonooka Road Moonbi 29 August 2007 Declaration of Restricted Area – Anambah 29 August 2007 Declaration of Restricted Area – Muswellbrook 29 August 2007 Declaration of Restricted Area – Aberdeen 29 August 2007 Declaration of Restricted Area – East Maitland 29 August 2007 Declaration of Restricted Area – Timbumburi 29 August 2007 Declaration of Restricted Area – McCullys Gap 30 August 2007 Declaration of Restricted Area – Bunnan 31 August 2007 Declaration of Restricted Area - Gloucester 31 August 2007 Declaration of Restricted Area – Eagleton 29 August 2007 SCHEDULE 2 The area shown in the map below and within the local government areas administered by the following councils: Cessnock City Council Dungog Shire Council Gloucester Shire Council Great Lakes Council Liverpool Plains Shire Council 6836 SPECIAL SUPPLEMENT 3 September 2007 Maitland City Council Muswellbrook Shire Council Newcastle City Council Port Stephens Council Singleton Shire Council Tamworth City Council Upper Hunter Shire Council NEW SOUTH WALES GOVERNMENT GAZETTE No. -

The History of the Worimi People by Mick Leon
The History of the Worimi People By Mick Leon The Tobwabba story is really the story of the original Worimi people from the Great Lakes region of coastal New South Wales, Australia. Before contact with settlers, their people extended from Port Stephens in the south to Forster/Tuncurry in the north and as far west as Gloucester. The Worimi is made up of several tribes; Buraigal, Gamipingal and the Garawerrigal. The people of the Wallis Lake area, called Wallamba, had one central campsite which is now known as Coomba Park. Their descendants, still living today, used this campsite 'til 1843. The Wallamba had possibly up to 500 members before white contact was made. The middens around the Wallis Lake area suggest that food from the lake and sea was abundant, as well as wallabies, kangaroos, echidnas, waterfowl and fruit bats. Fire was an important feature of life, both for campsites and the periodic 'burning ' of the land. The people now number less than 200 and from these families, in the main, come the Tobwabba artists. In their work, they express images of their environment, their spiritual beliefs and the life of their ancestors. The name Tobwabba means 'a place of clay' and refers to a hill on which the descendants of the Wallamba now have their homes. They make up a 'mission' called Cabarita with their own Land Council to administer their affairs. Aboriginal History of the Great Lakes District The following extract is provided courtesy of Great Lakes Council (Narelle Marr, 1997): In 1788 there were about 300,000 Aborigines in Australia. -

Part I General Geology
1 PART I GENERAL GEOLOGY GENERAL GEOLOGY Chapter 1 Introduction The areadescribed in this thesis, covering about 1500 square miles of the southern New England Tableland, lies within the Central Complex of north- eastern New South Wales, (Voisey, 1959), a belt of strongly deformed Palaeozoic sediments invaded by granitic intrusions of predominantly Permian age (see Fig. 1). Two major subdivisions have been made within the regionally metamorphosed rocks of this area, (see Map 1). Multi-folded, poly-metamorphosed rocks, herein named the Tia Complex underly a major portion of the area. These are intruded by a relatively small foliated pluton, the Tia Granodiorite, which occupies a more or less central position within these metamorphosed rocks and belongs to the Hillgrove Plutonic Suite of Binns et al (1967). In this respect the Tia Complex resembles the Wongwibinda Complex (Binns, 1966) lying some 70 miles to the north-north east. The other major subdivision consisting of sheared greywackes, slates and quartzites with some basic lavas occupies the area north-east and east of the Tia Complex. FIG, I. 3 Based on the Geological Map of N.S.W. (1962) with Tertiary Basalt omitted for clarity. • QLD. CLARENCE GREAT MORTON ARTESIAN + + + + + BASIN BASIN • • N.S.W. +++++ WongorthIndo Conte 0 TAMWORTH Argo descrilvd 13,11/1 mis trxc. O • • sre)Cr*aks,4. Ak,PORT MACQUARIE \MEND OC/N LORNE 1,+ BASIN MESOZOIC NEW ENGLAND BATNYL/7W [ + +I kel N/LLGROVE PLUTONIC SUITE rti SERPENT/WE 4 Progressive regional metamorphism in part of this area is spatially associated with granitic intrusions of the Moona Plains district, located in the north- east corner of the mapped area. -

(Phascolarctos Cinereus) on the North Coast of New South Wales
A Blueprint for a Comprehensive Reserve System for Koalas (Phascolarctos cinereus) on the North Coast of New South Wales Ashley Love (President, NPA Coffs Harbour Branch) & Dr. Oisín Sweeney (Science Officer, NPA NSW) April 2015 1 Acknowledgements This proposal incorporates material that has been the subject of years of work by various individuals and organisations on the NSW north coast, including the Bellengen Environment Centre; the Clarence Environment Centre; the Nambucca Valley Conservation Association Inc., the North Coast Environment Council and the North East Forest Alliance. 2 Traditional owners The NPA acknowledges the traditional Aboriginal owners and original custodians of the land mentioned in this proposal. The proposal seeks to protect country in the tribal lands of the Bundjalung, Gumbainggir, Dainggatti, Biripi and Worimi people. Citation This document should be cited as follows: Love, Ashley & Sweeney, Oisín F. 2015. A Blueprint for a comprehensive reserve system for koalas (Phascolarctos cinereus) on the North Coast of New South Wales. National Parks Association of New South Wales, Sydney. 3 Table of Contents Acknowledgements ....................................................................................................................................... 2 Traditional owners ........................................................................................................................................ 3 Citation ......................................................................................................................................................... -

Historical Riparian Vegetation Changes in Eastern NSW
University of Wollongong Research Online Faculty of Science, Medicine & Health - Honours Theses University of Wollongong Thesis Collections 2016 Historical Riparian Vegetation Changes in Eastern NSW Angus Skorulis Follow this and additional works at: https://ro.uow.edu.au/thsci University of Wollongong Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: This work is copyright. Apart from any use permitted under the Copyright Act 1968, no part of this work may be reproduced by any process, nor may any other exclusive right be exercised, without the permission of the author. Copyright owners are entitled to take legal action against persons who infringe their copyright. A reproduction of material that is protected by copyright may be a copyright infringement. A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. Unless otherwise indicated, the views expressed in this thesis are those of the author and do not necessarily represent the views of the University of Wollongong. Recommended Citation Skorulis, Angus, Historical Riparian Vegetation Changes in Eastern NSW, BSci Hons, School of Earth & Environmental Science, University of Wollongong, 2016. https://ro.uow.edu.au/thsci/120 Research Online is the open access institutional repository for the University of Wollongong. -

Midcoast Water
Who we are and what we do COMMUNITY INFORMATION BOOKLET 2016 Contents Introduction 3 MidCoast Water 4-5 Sustainable water cycle management 6 The water cycle 7 Our water supplies 8 The Manning Scheme 9-14 How does water get to our homes? 15 The treatment process 16-18 Other water supplies 19 Karuah River and Great Lakes Catchment 20 Water supply schemes 21-24 How much water do we use? 25 Let’s get waterwise 26 Don’t spray in the middle of the day! 27 Wastewater 28-31 Recycling 32 Wipes stop pipes 33 Think at the sink 34 Sewer spills 35 Water Quality Testing 36-37 Paying for it all 38-40 Does everyone have clean water? 41 For further information 42 2 Who we are and what we do Meet Whizzy: Introduction This is Whizzy the Waterdrop, MidCoast Water’s mascot. Whizzy Every day MidCoast Water cleans and pumps almost helps to remind us how 10 Olympic swimming pools worth of water through important it is to save a network of over a thousand kilometres of pipes to water and is a favourite of make sure that the people of the Manning, Great Lakes the children in our area. and Gloucester have ready access to safe water for all For more information on Whizzy email their needs. That water is used by almost 80 000 people community@ in 27 towns from Crowdy Head in the north, to Hawks midcoastwater.com.au Nest in the south, and Barrington in the west, before we take and treat the waste.