June 3, 2016 Karen B. Desalvo, M.D., M.P.H., M.Sc. Acting Assistant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bones of the Upper and Lower Limb
Bones of the Upper and Lower limb Musculoskeletal block- Anatomy-lecture 1 Editing file Objectives Color guide : important in Red ✓ Classify the bones of the three regions of the lower Doctor note in Green limb (thigh, leg and foot). Extra information in Grey ✓ Memorize the main features of the – Bones of the thigh (femur & patella) – Bones of the leg (tibia & Fibula) – Bones of the foot (tarsals, metatarsals and phalanges) ✓ Recognize the side of the bone. Note: this lecture is based on female slides since Prof abuel makarem said only things that are mentioned in the female slides will come in the exam Note : All bones picture which are described in this lecture are bones on the right side of the body Before start :Please make yourself familiar with these terms to better understand the lecture Terms Meaning Example Ridge The long and narrow upper edge, angle, or crest The supracondylar ridges (in the distal part of of something (the humerus The trochlear notch (in the proximal part of the Notch An indentation, (incision) on an edge or surface (ulna A nodule or a small rounded projection on the Tubercles (Dorsal tubercle (in the distal part of the radius bone A hollow place (The Notch is not complete but the Subscapular fossa (in the concave part of the Fossa fossa is complete and both of them act as the lock (scapula (of the joint A large prominence on a bone usually serving for Deltoid tuberosity (in the humorous) and it Tuberosity the attachment of muscles or ligaments (is a connects the deltoid muscle (bigger projection than the Tubercle -

Parts of the Body 1) Head – Caput, Capitus 2) Skull- Cranium Cephalic- Toward the Skull Caudal- Toward the Tail Rostral- Toward the Nose 3) Collum (Pl
BIO 3330 Advanced Human Cadaver Anatomy Instructor: Dr. Jeff Simpson Department of Biology Metropolitan State College of Denver 1 PARTS OF THE BODY 1) HEAD – CAPUT, CAPITUS 2) SKULL- CRANIUM CEPHALIC- TOWARD THE SKULL CAUDAL- TOWARD THE TAIL ROSTRAL- TOWARD THE NOSE 3) COLLUM (PL. COLLI), CERVIX 4) TRUNK- THORAX, CHEST 5) ABDOMEN- AREA BETWEEN THE DIAPHRAGM AND THE HIP BONES 6) PELVIS- AREA BETWEEN OS COXAS EXTREMITIES -UPPER 1) SHOULDER GIRDLE - SCAPULA, CLAVICLE 2) BRACHIUM - ARM 3) ANTEBRACHIUM -FOREARM 4) CUBITAL FOSSA 6) METACARPALS 7) PHALANGES 2 Lower Extremities Pelvis Os Coxae (2) Inominant Bones Sacrum Coccyx Terms of Position and Direction Anatomical Position Body Erect, head, eyes and toes facing forward. Limbs at side, palms facing forward Anterior-ventral Posterior-dorsal Superficial Deep Internal/external Vertical & horizontal- refer to the body in the standing position Lateral/ medial Superior/inferior Ipsilateral Contralateral Planes of the Body Median-cuts the body into left and right halves Sagittal- parallel to median Frontal (Coronal)- divides the body into front and back halves 3 Horizontal(transverse)- cuts the body into upper and lower portions Positions of the Body Proximal Distal Limbs Radial Ulnar Tibial Fibular Foot Dorsum Plantar Hallicus HAND Dorsum- back of hand Palmar (volar)- palm side Pollicus Index finger Middle finger Ring finger Pinky finger TERMS OF MOVEMENT 1) FLEXION: DECREASE ANGLE BETWEEN TWO BONES OF A JOINT 2) EXTENSION: INCREASE ANGLE BETWEEN TWO BONES OF A JOINT 3) ADDUCTION: TOWARDS MIDLINE -
Bones of the Upper and Lower Limb Musculoskeletal Block - Lecture 1
Bones of the Upper and Lower limb Musculoskeletal Block - Lecture 1 Objective: Classify the bones of the three regions of the lower limb (Thigh, leg and foot). Memorize the main features of the – Bones of the thigh (femur & patella) – Bones of the leg (tibia & Fibula) – Bones of the foot (tarsals, metatarsals and phalanges) Recognize the side of the bone. Color index: Important In male’s slides only In female’s slides only Extra information, explanation Editing file Click here for Contact us: useful videos [email protected] Please make sure that you’re familiar with these terms Terms Meaning Example Ridge The long and narrow upper edge, angle, or crest of something The supracondylar ridges (in the distal part of the humerus) Notch An indentation, (incision) on an edge or surface The trochlear notch (in the proximal part of the ulna) Tubercles A nodule or a small rounded projection on the bone (Dorsal tubercle in the distal part of the radius) Fossa A hollow place (The Notch is not complete but the fossa is Subscapular fossa (in the concave part of complete and both of them act as the lock of the joint the scapula) Tuberosity A large prominence on a bone usually serving for Deltoid tuberosity (in the humorous) and it the attachment of muscles or ligaments ( is a bigger projection connects the deltoid muscle than the Tubercle ) Processes A V-shaped indentation (act as the key of the joint) Coracoid process ( in the scapula ) Groove A channel, a long narrow depression sure Spiral (Radial) groove (in the posterior aspect of (the humerus -

Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur
Journal of Clinical Medicine Brief Report Intraosseous Bioplasty for a Subchondral Cyst in the Lateral Condyle of Femur Anish G.R. Potty 1,2,3, Ashim Gupta 1,4,5,6 , Hugo C. Rodriguez 1,2 , Ian W. Stone 2 and Nicola Maffulli 7,8,9,* 1 South Texas Orthopaedic Research Institute, Laredo, TX 78045, USA; [email protected] (A.G.R.P.); [email protected] (A.G.); [email protected] (H.C.R.) 2 School of Osteopathic Medicine, University of the Incarnate Word, San Antonio, TX 78209, USA; [email protected] 3 Laredo Sports Medicine Clinic, Laredo, TX 78041, USA 4 Department of Psychology, Illinois Wesleyan University, Bloomington, IL 61701, USA 5 Future Biologics, Lawrenceville, GA 30043, USA 6 BioIntegrate, Lawrenceville, GA 30043, USA 7 Department of Musculoskeletal Disorders, School of Medicine and Surgery, University of Salerno, 84084 Fisciano, Italy 8 Barts and the London School of Medicine and Dentistry, Centre for Sports and Exercise Medicine, Queen Mary University of London, London E1 4DG, UK 9 School of Pharmacy and Bioengineering, Keele University Faculty of Medicine, Stoke on Trent ST4 7QB, UK * Correspondence: n.maff[email protected] Received: 7 April 2020; Accepted: 4 May 2020; Published: 6 May 2020 Abstract: Several conditions can lead to the development of a subchondral cyst. The mechanism by which the cysts form, their location, and their severity depend on the underlying pathology, although the exact pathogenesis is not fully elucidated. Treatment options vary according to the location of the cyst, with less invasive procedures such as calcium phosphate cement injection to a joint arthroplasty when there is an extensive cyst in communication with the joint space. -

Species - Domesticus (Chicken) to Mammals (Human Being)
Int. J. LifeSc. Bt & Pharm. Res. 2013 Sunil N Tidke and Sucheta S Tidke, 2013 ISSN 2250-3137 www.ijlbpr.com Vol. 2, No. 4, October 2013 © 2013 IJLBPR. All Rights Reserved Research Paper MORPHOLOGY OF KNEE JOINT - CLASS- AVES - GENUS - GALLUS, - SPECIES - DOMESTICUS (CHICKEN) TO MAMMALS (HUMAN BEING) Sunil N Tidke1* and Sucheta S Tidke2 *Corresponding Author: Sunil N Tidke [email protected] In the present investigation, a detailed comparison is made between the human knee and the knee of chicken (Gallus domesticus), with the object of determining similarities or variation of structure and their possible functional significance, if any special attention has been paid to bone taking part in joint, the surrounding muscles and tendons, which play an important part in stabilizing these joints, the form and attachments of the intraarticular menisci, which have been credited with the function of ensuring efficient lubrication throughout joints movement, and to the ligaments, the function of which is disputed. Keywords: Bony articular part, Intra capsular and extra capsular structure and Muscular changes INTRODUCTION patella. A narrow groove on the lateral condyle of femur articulate with the head of the fibula and The manner in which the main articulations of intervening femoro fibular disc. The tibia has a the vertebrate have become variously modified enormous ridge and crest for the insertion of the in relation to diverse function has been patellar tendon and origin of the extensor muscle. investigated by many workers, notably, Parsons The cavity of the joint communicates above and (1900) and Haines (1942). The morphology of the below the menisci with the central part of joint knee joint of human has been studied in great around the cruciate ligament. -

Lab Activity 9
Lab Activity 9 Appendicular Skeleton Martini Chapter 8 Portland Community College BI 231 Appendicular Skeleton • Upper & Lower extremities • Shoulder Girdle • Pelvic Girdle 2 Humerus 3 Humerus: Proximal End Greater tubercle Lesser tubercle Head: Above the epiphyseal line Anatomical Neck Surgical neck Intertubercular groove Anterior Medial Posterior4 Deltoid Tuberosity 5 Radial Groove 6 Trochlea (Distal Humerus) Anterior Posterior Anterior Posterior 7 Capitulum (Distal Humerus) Anterior Posterior Anterior Posterior 8 Olecranon Fossa (Distal Humerus) Anterior Posterior Anterior Posterior 9 Medial Epicondyle (Distal Humerus) Anterior Posterior Anterior Posterior 10 Lateral Epicondyle (Distal Humerus) Anterior Posterior Anterior Posterior 11 Radial Fossa (Distal Humerus) Anterior Posterior Anterior Posterior 12 Coronoid Fossa (Distal Humerus) Anterior Posterior Anterior Posterior 13 Lateral Supracondylar Ridge (Distal Humerus) Anterior Posterior Anterior Posterior 14 Medial Supracondylar Ridge (Distal Humerus) Anterior Posterior Anterior Posterior 15 Humerus: Distal End/Anterior Medial Lateral Supracondylar Supracondylar Ridge Ridge Coronoid Fossa Radial fossa Lateral Medial Epicondyle Epicondyle Capitulum Trochlea 16 Humerus: Distal End/Posterior Olecranon Fossa Medial Epicondyle Lateral Epicondyle Trochlea 17 Radius • “Rotates” • On the thumb side of the forearm 18 Radius: Head 19 Radial Tuberosity 20 Ulnar Notch of the Radius 21 Ulnar Notch of the Radius 22 Radius: Interosseous Ridge 23 Styloid Process of the Radius 24 Radius Distal Anterior -
Case Report Osteochondral Fracture of the Lateral Femoral Condyle
Case Report Osteochondral fracture of the Lateral Femoral Condyle involving the entire weight bearing articular surface fixed with biodegradable screws Shah Jehan,1 Mark David Loeffler,2 Hamayon Pervez3 Hull Royal Infirmary, Hull,1 Colchester General Hospital, Colchester,2,3 United Kingdom. Abstract Osteochondral fracture of lateral femoral condyle can result from support and other twisting injuries of the knee. Arthroscopy is a better diagnostic and therapeutic tool as standard radiographs can mislead regarding the size and location of the fragment. If fragment is large and displaced, arthrotomy may become necessary. We present a case of large osteochondral fracture of lateral femoral condyle involving the entire articular surface in Figure-2: Large osteochondral fragment comprising the entire articular surface (left a 12 year old male. This was treated with open reduction side figure) reattached to lateral condyle of femur and fixed with four biodegradable and internal fixation using biodegradable screws. MRI at screws (right side figure). six weeks after surgery showed satisfactory results. After the surgery full weight bearing was allowed at follow up was arranged. When reviewed in the clinic one three months. week later an oblique radiograph was taken which showed a significant osteochondral fracture of the lateral femoral Case Report condyle. A twelve year old boy sustained a twisting injury to On arthroscopy of the knee there was a large defect his left knee whilst playing football. He presented to the in the articular surface of the lateral femoral condyle emergency department with severe pain and a large effusion involving the entire weight bearing area. A corresponding in the knee. -

Locking Plate Alone Or in Combination with Cannulated Screws for Hoffa Fractures: a Retrospective Cohort Study
Locking Plate alone or in Combination with Cannulated Screws for Hoffa Fractures: A retrospective cohort study Zhen Li Guangzhou University of Chinese Medicine Zhenyue Chen Guangzhou University of Chinese Medicine Xiaotan Wang Shandong University of Traditional Chinese Medicine Jingyin Li the First Aliated Hospital of Shandong University of Traditional Chinese Medicine Lizhong Jing the First Aliated Hospital of Shandong University of Traditional Chinese Medicine Xuewei Cao ( [email protected] ) Guangdong Provincial Hospital of Chinese Medicine Research Article Keywords: Hoffa fractures, locking plate, cannulated screws, early rehabilitation Posted Date: March 10th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-203399/v2 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/15 Abstract Purpose Locking plate or screws have been used for Hoffa fractures; however, evidence to support the effectiveness of the procedure remains scarce. The present study aimed to determine the ecacy of distal femur condyle locking plate(DFCLP)alone or in combination with cannulated screws for Hoffa fractures. Methods In this cohort study, 13 patients with isolated Hoffa fractures were enrolled during the study period (May 2014 to February 2019) and retrospectively analyzed. Patients underwent open reduction and internal xation by DFCLP alone or in combination with cannulated screws followed by early active rehabilitation postoperatively. The primary outcome was evaluated with Knee Society Score (KSS), the range of movement (ROM), International Knee Documentation Committee (IKDC) scoring system and the stability of the xation site of the patients during the 24-month follow-up period. Results A total of 13 patients completed the 24-month follow-up assessment and achieve bone re-union at Hoffa fracture sites. -

Species – Armiger (Bat) to Mammals (Human Being)
Int. J. LifeSc. Bt & Pharm. Res. 2013 Sunil N Tidke et al., 2013 ISSN 2250-3137 www.ijlbpr.com Vol. 2, No. 2, April 2013 © 2013 IJLBPR. All Rights Reserved Research Paper MORPHOLOGY OF KNEE JOINT OF TETRAPOD – CLASS MAMMALIA – GENUS – HIPPOSIDERUS – SPECIES – ARMIGER (BAT) TO MAMMALS (HUMAN BEING) Sunil N Tidke1* Bichitra N Roul1, Sucheta S Tidke2 and Mamita Nayak3 *Corresponding Author: Sunil N Tidke, [email protected] Advancement in knowledge of the comparative anatomy of joints has generally lagged behind than that of other structural systems. The knee joint has been chosen for present study as it represents the largest and functionally important articular unit, provided with an extensive synovial cavity and a variety of both intra and extra articular structures.The knee joint is of peculiar interest as manifesting a change of mechanism of locomotion in passing from tetrapods (Bat) to mammals and affording a means of studying, the corresponding modifications of anatomical structure. 10 bats armiger were selected and 10 human knee joints were selected from dissection hall in Anatomy department of Hi-Tech Medical College, Rourkela (Odisha). McMinn HMR has mentioned (5) that the bat is the only mammal which does not possess the menisci and popliteus muscle because it does not rotate the knee joint. The femorotibial articulation has both internal and external ligamentous connections. There is a single broad intra articular ligament which. may represent the initial form of the crucial ligaments and fibula merged with tibia. In the bat leg, the tibia and fibula are fused together. Keywords: Bony articular part, Intra capsular and extra capsular structures and Muscular changes INTRODUCTION situated near the center of gravity in contact to In this study of chordate skeletal anatomy it was forelimb. -

Anatomy and Physiology of Knee Stability
Journal of Functional Morphology and Kinesiology Review Anatomy and Physiology of Knee Stability Jawad F. Abulhasan 1,* and Michael J. Grey 2 1 Physiotherapy Department, Shaikhan Al-Faresi Hospital, Kuwait Ministry of Health, Kuwait City 44007, Kuwait 2 Acquired Brain Injury Rehabilitation Alliance, School of Health Sciences, University of East Anglia, Norwich NR4 7TJ, UK; [email protected] * Correspondence: [email protected]; Tel.: +965-6666-7770 Received: 28 June 2017; Accepted: 20 September 2017; Published: 24 September 2017 Abstract: Knee instability has been the focus of large number of studies over the last decade; however, a high incidence rate of injury still exists. The aim of this short report is to examine knee joint anatomy and physiology with respect to knee stability. Knee joint stability requires the integration of a complex set of anatomical structures and physiological mechanism. Compromising any of these structures leads to destabilisation and increased risk of injuries. This review highlights the structure and soft tissue of the knee that contribute to its stability and function. This introduction is part of the Journal of Functional Morphology and Kinesiology’s Special Issue “The Knee: Structure, Function and Rehabilitation”. Keywords: knee; anatomy; stability 1. Introduction Joint instability is a problem from which both athletes and non-athletes suffer, with one of the most common sources of instability being associated with the knee joint. Knee instability has a high incidence rate and has been extensively studied over the last decade. For example, one prospective cohort study conducted over seven consecutive professional football seasons found that injuries due to knee instability was second only to thigh strains (23%), and 18% of all injuries were sustained at the knee joint [1]. -
Bones of the Appendicular Skeleton
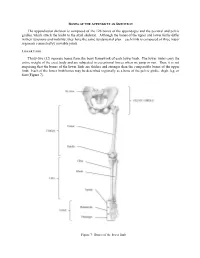
BONES OF THE APPENDICULAR SKELETON The appendicular skeleton is composed of the 126 bones of the appendages and the pectoral and pelvic girdles, which attach the limbs to the axial skeleton. Although the bones of the upper and lower limbs differ in their functions and mobility, they have the same fundamental plan – each limb is composed of three major segments connected by movable joints. LOWER LIMB Thirty-two (32) separate bones form the bony framework of each lower limb. The lower limbs carry the entire weight of the erect body and are subjected to exceptional forces when we jump or run. Thus, it is not surprising that the bones of the lower limb are thicker and stronger than the comparable bones of the upper limb. Each of the lower limb bones may be described regionally as a bone of the pelvic girdle, thigh, leg, or foot (Figure 7). Figure 7: Bones of the lower limb Pelvic (Hip) Girdle (Marieb / Hoehn – Chapter 7; Pgs. 234 – 238) The pelvic girdle is formed by the paired os coxae (coxal bones). Together with the sacrum and coccyx of the axial skeleton, this group of bones forms the bony pelvis. The ability to bear weight is more important in the pelvic girdle than the pectoral girdle. Thus, the os coxae are heavy and massive with a firm attachment to the axial skeleton. Each os coxa is a result of the fusion of three bones: the ilium, ischium, and pubis. These three bones fuse at the deep hemispherical socket, the acetabulum, which receives the femur. Figure 8: Right os coxa, lateral and medial views A. -

Lower Extremity Muscle Table
Robert Frysztak, PhD. Structure of the Human Body Loyola University Chicago Stritch School of Medicine LOWER EXTREMITY MUSCLE TABLE PROXIMAL ATTACHMENT DISTAL ATTACHMENT MUSCLE INNERVATION MAIN ACTIONS BLOOD SUPPLY MUSCLE GROUP (ORIGIN) (INSERTION) Lateral condyle of tibia, proximal 3/4 of Middle and distal phalanges of lateral Extends lateral four digits and Extensor digitorum longus anterior surface of interosseous Deep fibular nerve Anterior tibial artery Anterior leg four digits dorsiflexes foot at ankle membrane and fibula Middle part of anterior surface of fibula Dorsal aspect of base of distal phalanx Extends great toe, dorsiflexes foot at Extensor hallucis longus Deep fibular nerve Anterior tibial artery Anterior leg and interosseous membrane of great toe ankle Distal third of anterior surface of fibula Dorsiflexes foot at ankle and aids in Fibularis peroneus tertius Dorsum of base of 5th metatarsal Deep fibular nerve Anterior tibial artery Anterior leg and interosseous membrane eversion of foot Lateral condyle, proximal half of lateral Medial plantar surfaces of medial Dorsiflexes foot at ankle and inverts Tibialis anterior Deep fibular nerve Anterior tibial artery Anterior leg tibia, interosseous membrane cuneiform and base of 1st metatarsal foot Pulls suprapatellar bursa superiorly Articularis genus Distal femur on anterior surface Suprapatellar bursa Femoral nerve Femoral artery Anterior thigh with extension of knee First tendon into dorsal surface of base Aids the extensor digitorum longus in Superolateral surface of calcaneus,