Inherited Cataracts: Molecular Genetics, Clinical Features, Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
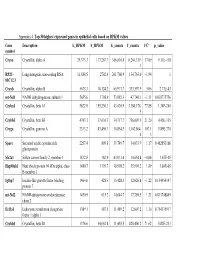
Appendix 4. Top 50 Highest Expressed Genes in Epithelial Cells Based on RPKM Values
Appendix 4. Top 50 highest expressed genes in epithelial cells based on RPKM values Gene Description E_RPKM F_RPKM E_counts F_counts FC* p_value symbol Cryaa Crystallin, alpha A 29,373.3 177,267.7 366,616.4 6,264,319. 17.09 9.11E-118 1 RP23– Long intergenic non-coding RNA 11,888.5 2702.4 261,760.9 134,763.0 −1.94 1 81C12.3 Cryab Crystallin, alpha B 5673.3 10,124.2 65,971.7 333,597.9 5.06 2.71E-43 mt-Nd1 NADH dehydrogenase, subunit 1 5655.6 1798.9 53,082.3 47,748.1 −1.11 0.838775756 Cryba1 Crystallin, beta A1 5622.0 155,230.3 43,420.9 3,380,176. 77.85 1.34E-240 5 Crybb3 Crystallin, beta B3 4743.1 37,636.3 34,717.7 736,007.9 21.20 4.45E-135 Cryga Crystallin, gamma A 2333.2 83,496.3 10,854.5 1,162,864. 107.1 5.89E-270 6 3 Sparc Secreted acidic cysteine rich 2257.4 809.8 39,749.7 34,033.9 −1.17 0.462853166 glycoprotein Slc2a1 Solute carrier family 2, member 1 1832.8 162.9 43,031.4 10,654.8 −4.04 1.67E-05 Hsp90ab1 Heat shock protein 90 kDa alpha, class 1480.7 1139.7 18,998.2 35,901.2 1.89 3.84E-05 B member 1 Igfbp7 Insulin-like growth factor binding 1464.6 428.3 15,428.3 12,626.8 −1.22 0.154954147 protein 7 mt-Nd2 NADH-ubiquinone oxidoreductase 1450.9 615.2 14,644.7 17,789.5 1.21 0.833748849 chain 2 Eef1a1 Eukaryotic translation elongation 1389.1 587.5 11,489.2 12,607.2 1.10 0.754135917 factor 1 alpha 1 Crybb1 Crystallin, beta B1 1376.6 34,662.8 11,455.5 820,406.2 71.62 5.82E-233 Htra3 HtrA serine peptidase 3 1338.6 162.0 23,197.6 6433.9 −3.61 3.93E-05 Gnb2l1 Guanine nucleotide-binding protein 1293.3 670.1 14,495.1 21,652.1 1.49 0.001685952 -

CRYGB Rabbit Pab
Leader in Biomolecular Solutions for Life Science CRYGB Rabbit pAb Catalog No.: A14569 Basic Information Background Catalog No. Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. A14569 The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their Observed MW nuclei during development, these crystallins are made and then retained throughout 21kDa life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a Calculated MW superfamily. Alpha and beta families are further divided into acidic and basic groups. 20kDa Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Gamma-crystallins are a homogeneous group of Category highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. Four Primary antibody gamma-crystallin genes (gamma-A through gamma-D) and three pseudogenes (gamma-E, gamma-F, gamma-G) are tandemly organized in a genomic segment as a Applications gene cluster. Whether due to aging or mutations in specific genes, gamma-crystallins WB have been involved in cataract formation. Cross-Reactivity Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 1419 P07316 Immunogen Recombinant fusion protein containing a sequence corresponding to amino acids 80-135 of human CRYGB (NP_005201.2). Synonyms CRYGB;CRYG2;CTRCT39 Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. -

Pediatric Cataract
Educational Article Pediatric cataract Α. Νikolaidou, T. Chatzibalis ABSTRACT INTRODUCTION Pediatric cataract constitutes one amongst the leading Cataract is an opacification of the crystalline lens of the causes of childhood blindness. Blindness due to pediatric eye that can result in blindness if not treated soon enough, cataract can be treated with early identification and thought- ful management. When left untreated, cataract in children partially or totally. For children, cataracts of a wide etiology can result in social and economic hurdles for the child but constitute a common cause of blindness, developing often also for society. Hence, the early diagnosis followed by slowly laterally or bilaterally. Early signs of cataract can oc- prompt treatment is of great significance. Routine screening cur as blurry or double vision, halos around light, trouble usually leads to diagnosis while some cases may be referred seeing at night or with bright lighting and faded colors while after parents notice of leukocoria or strabismus. Etiology of parents usually point out leukocoria or strabismus. Timely pediatric cataract is widely miscellaneous and diagnosis of specific etiology assists in effective management. Consider- identification and intervention are of critical significance for 1 ing therapy, pediatric cataract surgery has evolved, by im- a favorable visual outcome. proving knowledge of myopic shift and axial length growth, with the implementation of IOLs being in the spotlight. The number of procedures for IOL implantations increases stead- EPIDEMIOLOGY ily every year. Favorable results depend not only on effective surgery, but also on postoperative care and rehabilitation. Nevertheless, parents, surgeons, anesthesiologists, pedia- The prevalence of childhood cataracts ranges extensively tricians, and optometrists need to work together in order to in the reports due to differences in populations, definition achieve desirable outcomes. -

Supp Table 1.Pdf
Upregulated genes in Hdac8 null cranial neural crest cells fold change Gene Symbol Gene Title 134.39 Stmn4 stathmin-like 4 46.05 Lhx1 LIM homeobox protein 1 31.45 Lect2 leukocyte cell-derived chemotaxin 2 31.09 Zfp108 zinc finger protein 108 27.74 0710007G10Rik RIKEN cDNA 0710007G10 gene 26.31 1700019O17Rik RIKEN cDNA 1700019O17 gene 25.72 Cyb561 Cytochrome b-561 25.35 Tsc22d1 TSC22 domain family, member 1 25.27 4921513I08Rik RIKEN cDNA 4921513I08 gene 24.58 Ofa oncofetal antigen 24.47 B230112I24Rik RIKEN cDNA B230112I24 gene 23.86 Uty ubiquitously transcribed tetratricopeptide repeat gene, Y chromosome 22.84 D8Ertd268e DNA segment, Chr 8, ERATO Doi 268, expressed 19.78 Dag1 Dystroglycan 1 19.74 Pkn1 protein kinase N1 18.64 Cts8 cathepsin 8 18.23 1500012D20Rik RIKEN cDNA 1500012D20 gene 18.09 Slc43a2 solute carrier family 43, member 2 17.17 Pcm1 Pericentriolar material 1 17.17 Prg2 proteoglycan 2, bone marrow 17.11 LOC671579 hypothetical protein LOC671579 17.11 Slco1a5 solute carrier organic anion transporter family, member 1a5 17.02 Fbxl7 F-box and leucine-rich repeat protein 7 17.02 Kcns2 K+ voltage-gated channel, subfamily S, 2 16.93 AW493845 Expressed sequence AW493845 16.12 1600014K23Rik RIKEN cDNA 1600014K23 gene 15.71 Cst8 cystatin 8 (cystatin-related epididymal spermatogenic) 15.68 4922502D21Rik RIKEN cDNA 4922502D21 gene 15.32 2810011L19Rik RIKEN cDNA 2810011L19 gene 15.08 Btbd9 BTB (POZ) domain containing 9 14.77 Hoxa11os homeo box A11, opposite strand transcript 14.74 Obp1a odorant binding protein Ia 14.72 ORF28 open reading -

A Comprehensive Analysis of the Expression of Crystallins in Mouse Retina Jinghua Xi Washington University School of Medicine in St
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2003 A comprehensive analysis of the expression of crystallins in mouse retina Jinghua Xi Washington University School of Medicine in St. Louis Rafal Farjo University of Michigan - Ann Arbor Shigeo Yoshida University of Michigan - Ann Arbor Timothy S. Kern Case Western Reserve University Anand Swaroop University of Michigan - Ann Arbor See next page for additional authors Follow this and additional works at: https://digitalcommons.wustl.edu/open_access_pubs Recommended Citation Xi, Jinghua; Farjo, Rafal; Yoshida, Shigeo; Kern, Timothy S.; Swaroop, Anand; and Andley, Usha P., ,"A comprehensive analysis of the expression of crystallins in mouse retina." Molecular Vision.9,. 410-419. (2003). https://digitalcommons.wustl.edu/open_access_pubs/1801 This Open Access Publication is brought to you for free and open access by Digital Commons@Becker. It has been accepted for inclusion in Open Access Publications by an authorized administrator of Digital Commons@Becker. For more information, please contact [email protected]. Authors Jinghua Xi, Rafal Farjo, Shigeo Yoshida, Timothy S. Kern, Anand Swaroop, and Usha P. Andley This open access publication is available at Digital Commons@Becker: https://digitalcommons.wustl.edu/open_access_pubs/1801 Molecular Vision 2003; 9:410-9 <http://www.molvis.org/molvis/v9/a53> © 2003 Molecular Vision Received 28 May 2003 | Accepted 19 August 2003 | Published 28 August 2003 A comprehensive analysis of the expression of crystallins in mouse retina Jinghua Xi,1 Rafal Farjo,3 Shigeo Yoshida,3 Timothy S. Kern,5 Anand Swaroop,3,4 Usha P. Andley1,2 Departments of 1Ophthalmology and Visual Sciences and 2Biochemistry and Molecular Biophysics, Washington University School of Medicine, St. -

Related Macular Degeneration and Cutis Laxa
UvA-DARE (Digital Academic Repository) Genetic studies of age-related macular degeneration Baas, D.C. Publication date 2012 Document Version Final published version Link to publication Citation for published version (APA): Baas, D. C. (2012). Genetic studies of age-related macular degeneration. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:05 Oct 2021 G������ S������ �� A��-������� M������ D����������� D����������� M������ G������ S������ �� A��-������� | 2012 D�������� C. B��� G������ S������ �� A��-������� M������ D����������� D�������� C. B��� cover.indd 1 31-10-12 08:36 Genetic Studies of Age-related Macular Degeneration Dominique C. Baas Chapter 0.indd 1 23-10-12 19:24 The research described in this thesis was conducted at the Netherlands Institute for Neuroscience (NIN), an institute of the Royal Netherlands Academy of Arts and Sciences, Department of Clinical and Molecular Ophthalmogenetics, Amsterdam, The Netherlands. -

Congenital Cataracts Due to a Novel 2‑Bp Deletion in CRYBA1/A3
1614 MOLECULAR MEDICINE REPORTS 10: 1614-1618, 2014 Congenital cataracts due to a novel 2‑bp deletion in CRYBA1/A3 JING ZHANG1, YANHUA ZHANG1, FANG FANG1, WEIHONG MU1, NING ZHANG2, TONGSHUN XU3 and QINYING CAO1 1Prenatal Diagnosis Center, Shijiazhuang Obstetrics and Gynecology Hospital; 2Department of Cardiology, The Second Hospital of Hebei Medical University; 3Department of Surgery, Shijiazhuang Obstetrics and Gynecology Hospital, Shijiazhuang, Hebei, P.R. China Received September 22, 2013; Accepted April 11, 2014 DOI: 10.3892/mmr.2014.2324 Abstract. Congenital cataracts, which are a clinically and located in the eye lens. The major human crystallins comprise genetically heterogeneous group of eye disorders, lead to 90% of protein in the mature lens and contain two different visual impairment and are a significant cause of blindness superfamilies: the small heat‑shock proteins (α-crystallins) in childhood. A major proportion of the causative mutations and the βγ-crystallins. for congenital cataracts are found in crystallin genes. In the In this study a functional candidate approach was used present study, a novel deletion mutation (c.590-591delAG) in to investigate the known crystallin genes, including CRYAA, exon 6 of CRYBA1/A3 was identified in a large family with CRYAB, CRYBA1/A3, CRYBB1, CRYBB2, CRYGC, CRYGD autosomal dominant congenital cataracts. An increase in and CRYGS, in which a major proportion of the mutations local hydrophobicity was predicted around the mutation site; identified in a large family with congenital cataracts were however, further studies are required to determine the exact found. effect of the mutation on βA1/A3-crystallin structure and function. To the best of our knowledge, this is the first report Subjects and methods of an association between a frameshift mutation in exon 6 of CRYBA1/A3 and congenital cataracts. -

Congenital Cataract- Approach and Management Review
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 16, Issue 5 Ver. V (May. 2017), PP 56-61 www.iosrjournals.org Congenital Cataract- Approach and Management Review Dr.Bhawesh Chandra Saha1, Dr.Rashmi Kumari2, Dr Bibhuti Prasanna Sinha3 1Senior Resident,AIIMS ,Patna 2Senior Resident,Regional Institute Of Ophthalmology,IGIMS ,Patna 3Professor,IGIMS,Patna All India Institute Of Medical Sciences ,Patna ,Indira Gandhi Institute Of Medical Sciences,Patna Abstract: Childhood cataract remains a challenge to pediatric ophthalmologists despite recent major breakthrough in surgical techniques and instrumentation. Pediatric cataract is one of the major causes of preventable childhood blindness, affecting approximately 200,000 children worldwide, with an estimated prevalence ranging from three to six per 10,000 live births Congenital cataracts usually are diagnosed at birth. If a cataract goes undetected in an infant, permanent visual loss may ensue. The management of pediatric cataract is a team effort of ophthalmologist ,pediatrician,anaesthetist and parents and should be customized depending upon the age of onset, laterality, morphology of the cataract, and other associated ocular and systemic co-morbidities.This review attempts to summarize the available management options to these patients along with some analytical recommendations to optimise the outcome. Keywords: Pediatric cataract,childhood blindness I. Introduction Pediatric cataract is one of the major causes of preventable childhood blindness, affecting approximately 200,000 children worldwide, with an estimated prevalence ranging from three to six per 10,000 live births1-3. It may be congenital, if present within the first year of life, developmental if present after infancy, or traumatic. -

Outcomes of Pediatric Cataract Surgery at a Tertiary Care Center in Rural Southern Ethiopia
CLINICAL SCIENCES Outcomes of Pediatric Cataract Surgery at a Tertiary Care Center in Rural Southern Ethiopia Oren Tomkins, MD, PhD; Itay Ben-Zion, MD; Daniel B. Moore, MD; Eugene E. Helveston, MD Objective: To evaluate the etiologies, management, and (n=33), congenital glaucoma-related (n=3), partially ab- outcomes of pediatric cataracts in a rural sub-Saharan Afri- sorbed cataracts (n=3), and congenital rubella infec- can setting. tions (n=2). At presentation, visual acuity ranged from 6/60 to light perception, with 13 eyes (14%) having am- Methods: A retrospective, consecutive case series of pa- bulatory vision (better than hand motion). The mean post- tients presenting to a tertiary referral center in southern operative visual acuity was significantly improved, rang- Ethiopia during a 13-month period. All patients under- ing from light perception to 6/9. Seventy-five eyes (82%) went clinical examination, were diagnosed as having cata- achieved ambulatory vision. Of the 61 eyes with an im- ract on the basis of standard clinical assessment, and im- planted intraocular lens, 56 (92%) reached ambulatory mediately underwent surgical management. Visual acuity visual acuity following surgery. This was significantly results were grossly divided into ambulatory and non- greater than preoperative visual acuity results (PϽ.001). ambulatory vision according to patient age and coopera- tion. Conclusions: The underlying cause and management of pediatric cataracts in the developing world can differ sig- Results: Ninety-one eyes of 73 consecutive patients (57 nificantly from that commonly reported in the litera- boys and 16 girls) were included in the study. The mean ture. The effects of appropriate intervention on both vi- (SEM) age at diagnosis was 7.1(0.5) years (range, 0.5-15 sual outcome and associated survival statistics may be years). -

Pediatric Cataracts: a Retrospective Study of 12 Years (2004
Pediatric Cataracts: A Retrospective Study of 12 Years (2004 - 2016) Cataratas em Idade Pediátrica: Estudo Retrospetivo de 12 ARTIGO ORIGINAL Anos (2004 - 2016) Jorge MOREIRA1, Isabel RIBEIRO1, Ágata MOTA1, Rita GONÇALVES1, Pedro COELHO1, Tiago MAIO1, Paula TENEDÓRIO1 Acta Med Port 2017 Mar;30(3):169-174 ▪ https://doi.org/10.20344/amp.8223 ABSTRACT Introduction: Cataracts are a major cause of preventable childhood blindness. Visual prognosis of these patients depends on a prompt therapeutic approach. Understanding pediatric cataracts epidemiology is of great importance for the implementation of programs of primary prevention and early diagnosis. Material and Methods: We reviewed the clinical cases of pediatric cataracts diagnosed in the last 12 years at Hospital Pedro Hispano, in Porto. Results: We identified 42 cases of pediatric cataracts with an equal gender distribution. The mean age at diagnosis was 6 years and 64.3% of patients had bilateral disease. Decreased visual acuity was the commonest presenting sign (36.8%) followed by leucocoria (26.3%). The etiology was unknown in 59.5% of cases and there was a slight predominance of nuclear type cataract (32.5%). Cataract was associated with systemic diseases in 23.8% of cases and with ocular abnormalities in 33.3% of cases. 47.6% of patients were treated surgically. Postoperative complications occurred in 35% of cases and posterior capsular opacification was the most common (25%). Discussion: The report of 42 cases is probably the result of the low prevalence of cataracts in this age. Although the limitations of our study include small sample size, the profile of children with cataracts in our hospital has characteristics relatively similar to those described in the literature. -

Congenital Eye Disorders Gene Panel
Congenital eye disorders gene panel Contact details Introduction Regional Genetics Service Ocular conditions are highly heterogeneous and show considerable phenotypic overlap. 1 in Levels 4-6, Barclay House 2,500 children in the UK are diagnosed as blind or severely visually impaired by the time they 37 Queen Square reach one year old. As many as half of these cases are likely to be inherited and remain undiagnosed due to the vast number of genes involved in these conditions. Many congenital London, WC1N 3BH eye disorders causing visual impairment or blindness at birth or progressive visual impairment T +44 (0) 20 7762 6888 also include syndromic conditions involving additional metabolic, developmental, physical or F +44 (0) 20 7813 8578 sensory abnormalities. Gene panels offer the enhanced probability of diagnosis as a very large number of genes can be interrogated. Samples required Ocular birth defects include all inheritance modalities. Autosomal dominant and recessive 5ml venous blood in plastic EDTA diseases as well as X-linked dominant and recessive diseases are seen. These conditions can bottles (>1ml from neonates) also be caused by de novo variants. Prenatal testing must be arranged Referrals in advance, through a Clinical Genetics department if possible. Patients presenting with a phenotype appropriate for the requested sub-panel Amniotic fluid or CV samples Referrals will be accepted from clinical geneticists and consultants in ophthalmology. should be sent to Cytogenetics for Prenatal testing dissecting and culturing, with instructions to forward the sample Prenatal diagnosis may be offered as appropriate where pathogenic variants have been to the Regional Molecular Genetics identified in accordance with expected inheritance pattern and where appropriate parental laboratory for analysis testing and counselling has been conducted. -

Clinical Study of Paediatric Cataract and Visual Outcome After Iol Implantation
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 18, Issue 5 Ser. 13 (May. 2019), PP 01-05 www.iosrjournals.org Clinical Study of Paediatric Cataract and Visual Outcome after Iol Implantation Dr. Dhananjay Prasad1,Dr. Vireshwar Prasad2 1(SENIOR RESIDENT) Nalanda Medical College and Hospital, Patna 2(Ex. HOD and Professor UpgradedDepartment of Eye, DMCH Darbhanga) Corresponding Author:Dr. Dhananjay Prasad Abstract: Objectives: (1) To know the possible etiology of Paediatric cataract, (2)Type of Paediatric cataract (3)Associated other ocular abnormality (microophtalmia, nystagmus, Strabismus, Amblyopia, corneal opacity etc.), (4) Systemic association, (5) Laterality (whether unilateral or bilateral), (6) Sex incidence (7)Pre-operative vision (8) To evaluate the visual results after cataract surgery in children aged between 2-15 years and (9) To evaluate the complication and different causes of visual impairment following the management. ----------------------------------------------------------------------------------------------------------------------------- ---------- Date of Submission: 09-05-2019 Date of acceptance: 25-05-2019 ----------------------------------------------------------------------------------------------------------------------------- ---------- I. Material And Methods Prospective study was conducted in the Department of Ophthalmology at Darbhanga Medical College and Hospital, Laheriasarai (Bihar).The material for the present study was drawn from patients attending the out- patient Department of Ophthalmology for cataract management during the period from November 2012 to October 2014. 25 cases (40 Eyes) of pediatric cataract were included in the study. Patients were admitted and the data was categorized into etiology, age, and sex and analyzed. All the cases were studied in the following manner. Inclusion Criteria: • All children above 2 years of age and below 15 years with visually significant cataract.