V13a25-Kamphuis Pgmkr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
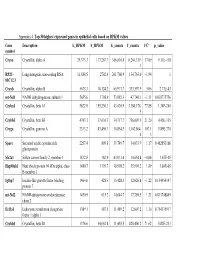
Appendix 4. Top 50 Highest Expressed Genes in Epithelial Cells Based on RPKM Values
Appendix 4. Top 50 highest expressed genes in epithelial cells based on RPKM values Gene Description E_RPKM F_RPKM E_counts F_counts FC* p_value symbol Cryaa Crystallin, alpha A 29,373.3 177,267.7 366,616.4 6,264,319. 17.09 9.11E-118 1 RP23– Long intergenic non-coding RNA 11,888.5 2702.4 261,760.9 134,763.0 −1.94 1 81C12.3 Cryab Crystallin, alpha B 5673.3 10,124.2 65,971.7 333,597.9 5.06 2.71E-43 mt-Nd1 NADH dehydrogenase, subunit 1 5655.6 1798.9 53,082.3 47,748.1 −1.11 0.838775756 Cryba1 Crystallin, beta A1 5622.0 155,230.3 43,420.9 3,380,176. 77.85 1.34E-240 5 Crybb3 Crystallin, beta B3 4743.1 37,636.3 34,717.7 736,007.9 21.20 4.45E-135 Cryga Crystallin, gamma A 2333.2 83,496.3 10,854.5 1,162,864. 107.1 5.89E-270 6 3 Sparc Secreted acidic cysteine rich 2257.4 809.8 39,749.7 34,033.9 −1.17 0.462853166 glycoprotein Slc2a1 Solute carrier family 2, member 1 1832.8 162.9 43,031.4 10,654.8 −4.04 1.67E-05 Hsp90ab1 Heat shock protein 90 kDa alpha, class 1480.7 1139.7 18,998.2 35,901.2 1.89 3.84E-05 B member 1 Igfbp7 Insulin-like growth factor binding 1464.6 428.3 15,428.3 12,626.8 −1.22 0.154954147 protein 7 mt-Nd2 NADH-ubiquinone oxidoreductase 1450.9 615.2 14,644.7 17,789.5 1.21 0.833748849 chain 2 Eef1a1 Eukaryotic translation elongation 1389.1 587.5 11,489.2 12,607.2 1.10 0.754135917 factor 1 alpha 1 Crybb1 Crystallin, beta B1 1376.6 34,662.8 11,455.5 820,406.2 71.62 5.82E-233 Htra3 HtrA serine peptidase 3 1338.6 162.0 23,197.6 6433.9 −3.61 3.93E-05 Gnb2l1 Guanine nucleotide-binding protein 1293.3 670.1 14,495.1 21,652.1 1.49 0.001685952 -

CRYGB Rabbit Pab
Leader in Biomolecular Solutions for Life Science CRYGB Rabbit pAb Catalog No.: A14569 Basic Information Background Catalog No. Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. A14569 The latter class constitutes the major proteins of vertebrate eye lens and maintains the transparency and refractive index of the lens. Since lens central fiber cells lose their Observed MW nuclei during development, these crystallins are made and then retained throughout 21kDa life, making them extremely stable proteins. Mammalian lens crystallins are divided into alpha, beta, and gamma families; beta and gamma crystallins are also considered as a Calculated MW superfamily. Alpha and beta families are further divided into acidic and basic groups. 20kDa Seven protein regions exist in crystallins: four homologous motifs, a connecting peptide, and N- and C-terminal extensions. Gamma-crystallins are a homogeneous group of Category highly symmetrical, monomeric proteins typically lacking connecting peptides and terminal extensions. They are differentially regulated after early development. Four Primary antibody gamma-crystallin genes (gamma-A through gamma-D) and three pseudogenes (gamma-E, gamma-F, gamma-G) are tandemly organized in a genomic segment as a Applications gene cluster. Whether due to aging or mutations in specific genes, gamma-crystallins WB have been involved in cataract formation. Cross-Reactivity Mouse, Rat Recommended Dilutions Immunogen Information WB 1:500 - 1:2000 Gene ID Swiss Prot 1419 P07316 Immunogen Recombinant fusion protein containing a sequence corresponding to amino acids 80-135 of human CRYGB (NP_005201.2). Synonyms CRYGB;CRYG2;CTRCT39 Contact Product Information www.abclonal.com Source Isotype Purification Rabbit IgG Affinity purification Storage Store at -20℃. -

Related Macular Degeneration and Cutis Laxa
UvA-DARE (Digital Academic Repository) Genetic studies of age-related macular degeneration Baas, D.C. Publication date 2012 Document Version Final published version Link to publication Citation for published version (APA): Baas, D. C. (2012). Genetic studies of age-related macular degeneration. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:05 Oct 2021 G������ S������ �� A��-������� M������ D����������� D����������� M������ G������ S������ �� A��-������� | 2012 D�������� C. B��� G������ S������ �� A��-������� M������ D����������� D�������� C. B��� cover.indd 1 31-10-12 08:36 Genetic Studies of Age-related Macular Degeneration Dominique C. Baas Chapter 0.indd 1 23-10-12 19:24 The research described in this thesis was conducted at the Netherlands Institute for Neuroscience (NIN), an institute of the Royal Netherlands Academy of Arts and Sciences, Department of Clinical and Molecular Ophthalmogenetics, Amsterdam, The Netherlands. -

Congenital Eye Disorders Gene Panel
Congenital eye disorders gene panel Contact details Introduction Regional Genetics Service Ocular conditions are highly heterogeneous and show considerable phenotypic overlap. 1 in Levels 4-6, Barclay House 2,500 children in the UK are diagnosed as blind or severely visually impaired by the time they 37 Queen Square reach one year old. As many as half of these cases are likely to be inherited and remain undiagnosed due to the vast number of genes involved in these conditions. Many congenital London, WC1N 3BH eye disorders causing visual impairment or blindness at birth or progressive visual impairment T +44 (0) 20 7762 6888 also include syndromic conditions involving additional metabolic, developmental, physical or F +44 (0) 20 7813 8578 sensory abnormalities. Gene panels offer the enhanced probability of diagnosis as a very large number of genes can be interrogated. Samples required Ocular birth defects include all inheritance modalities. Autosomal dominant and recessive 5ml venous blood in plastic EDTA diseases as well as X-linked dominant and recessive diseases are seen. These conditions can bottles (>1ml from neonates) also be caused by de novo variants. Prenatal testing must be arranged Referrals in advance, through a Clinical Genetics department if possible. Patients presenting with a phenotype appropriate for the requested sub-panel Amniotic fluid or CV samples Referrals will be accepted from clinical geneticists and consultants in ophthalmology. should be sent to Cytogenetics for Prenatal testing dissecting and culturing, with instructions to forward the sample Prenatal diagnosis may be offered as appropriate where pathogenic variants have been to the Regional Molecular Genetics identified in accordance with expected inheritance pattern and where appropriate parental laboratory for analysis testing and counselling has been conducted. -

Supplementary Materials
Supplementary materials Gene Nucleotide Amino acid change Clinical phenotype Ref symbol change CRYAA c.35G>T p. R12L lens protein gene [1] CRYAB c.32G>A p. R11H lens protein gene [2] CRYBA1 c.279-281delG p.ΔG91 lens protein gene [3] AG CRYBA4 c.206T>C p. L69P lens protein gene [4] CRYBB1 c.658G>T p. G220X lens protein gene [5] CRYBB2 c.563G>A p. R188H lens protein gene [6] CRYBB3 c.314G>A p. R105Q lens protein gene [7] CRYGA c.196T>C p. Y66H lens protein gene [8] CRYGB c.449G>T p. G150V lens protein gene [8] CRYGC c.385G>T p. G129C lens protein gene [9] CRYGD c.70C>A p. P24T lens protein gene [10] CRYGS c.53G>T :p.G18V lens protein gene [11] GJA3 c.188A>G p.N63S membrane protein [12] gene GJA8 c.262C>T p.P88S membrane protein [13] gene BFSP1 c736-1384_c.9 T246fsX7 cytoskeleton protein [14] 57-66del gene BFSP2 c.1091G>A p.G364D cytoskeleton protein [15] gene PAX6 c.307C>T p.R103X developmental [16] regulatory protein gen PITX3 c.38G>A p.S13N developmental [17] regulatory protein gen HSF4 c.524G>C p.R175P developmental [18] regulatory protein gen MAF c.863G>C p.R288P developmental [19] regulatory protein gen CHMP4B c.481G>A p. E161K chromatin modified [20] protein gene EPHA2 c.2842G>T p. G948W tyrosine kinase [21] receptor gene COL4A1 c.2345G>C p. G782A syndrome-related [22] genes FTL c.160G>A p.E54K developmental [23] regulatory protein gen GALK1 c.416T>C p. -

CRYGB (NM 005210) Human Tagged ORF Clone – RC214533L1
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC214533L1 CRYGB (NM_005210) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: CRYGB (NM_005210) Human Tagged ORF Clone Tag: Myc-DDK Symbol: CRYGB Synonyms: CRYG2; CTRCT39 Vector: pLenti-C-Myc-DDK (PS100064) E. coli Selection: Chloramphenicol (34 ug/mL) Cell Selection: None ORF Nucleotide The ORF insert of this clone is exactly the same as(RC214533). Sequence: Restriction Sites: SgfI-MluI Cloning Scheme: ACCN: NM_005210 ORF Size: 525 bp This product is to be used for laboratory only. Not for diagnostic or therapeutic use. View online » ©2021 OriGene Technologies, Inc., 9620 Medical Center Drive, Ste 200, Rockville, MD 20850, US 1 / 3 CRYGB (NM_005210) Human Tagged ORF Clone – RC214533L1 OTI Disclaimer: The molecular sequence of this clone aligns with the gene accession number as a point of reference only. However, individual transcript sequences of the same gene can differ through naturally occurring variations (e.g. polymorphisms), each with its own valid existence. This clone is substantially in agreement with the reference, but a complete review of all prevailing variants is recommended prior to use. More info OTI Annotation: This clone was engineered to express the complete ORF with an expression tag. Expression varies depending on the nature of the gene. RefSeq: NM_005210.2, NP_005201.1 RefSeq Size: 643 bp RefSeq ORF: 528 bp Locus ID: 1419 UniProt ID: P07316 Protein Families: Druggable Genome MW: 20.9 kDa Gene Summary: Crystallins are separated into two classes: taxon-specific, or enzyme, and ubiquitous. -

Novel Mutation in the Γ-S Crystallin Gene Causing Autosomal Dominant Cataract
Molecular Vision 2009; 15:476-481 <http://www.molvis.org/molvis/v15/a48> © 2009 Molecular Vision Received 24 December 2008 | Accepted 27 February 2009 | Published 4 March 2009 Novel mutation in the γ-S crystallin gene causing autosomal dominant cataract Vanita Vanita,1 Jai Rup Singh,1 Daljit Singh,2 Raymonda Varon,3 Karl Sperling3 1Centre for Genetic Disorders, Guru Nanak Dev University, Amritsar, India; 2Dr. Daljit Singh Eye Hospital, Amritsar, India; 3Institute of Human Genetics, Charitè, University Medicine of Berlin, Berlin, Germany Purpose: To identify the underlying genetic defect in a north Indian family with seven members in three-generations affected with bilateral congenital cataract. Methods: Detailed family history and clinical data were recorded. Linkage analysis using fluorescently labeled microsatellite markers for the already known candidate gene loci was performed in combination with mutation screening by bidirectional sequencing. Results: Affected individuals had bilateral congenital cataract. Cataract was of opalescent type with the central nuclear region denser than the periphery. Linkage was excluded for the known cataract candidate gene loci at 1p34–36, 1q21–25 (gap junction protein, alpha 8 [GJA8]), 2q33–36 (crystallin, gamma A [CRYGA], crystallin, gamma B [CRYGB], crystallin, gamma C [CRYGC], crystallin, gamma D [CRYGD], crystallin, beta A2 [CRYBA2]), 3q21–22 (beaded filament structural protein 2, phakinin [BFSP2]), 12q12–14 (aquaporin 0 [AQP0]), 13q11–13 (gap junction protein, alpha 3 [GJA3]), 15q21– 22, 16q22–23 (v-maf musculoaponeurotic fibrosarcoma oncogene homolog [MAF], heat shock transcription factor 4 [HSF4]), 17q11–12 (crystallin, beta A1 [CRYBA1]), 17q24, 21q22.3 (crystallin, alpha A [CRYAA]), and 22q11.2 (crystallin, beta B1 [CRYBB1], crystallin, beta B2 [CRYBB2], crystallin, beta B3 [CRYBB3], crystallin, beta A4 [CRYBA4]). -

Effects of Maternal Immune Activation on Gene Expression Patterns in the Fetal Brain
Citation: Transl Psychiatry (2012) 2, e98, doi:10.1038/tp.2012.24 & 2012 Macmillan Publishers Limited All rights reserved 2158-3188/12 www.nature.com/tp Effects of maternal immune activation on gene expression patterns in the fetal brain KA Garbett1,5, EY Hsiao2,5,SKa´lma´n1,3, PH Patterson2 and K Mirnics1,4 We are exploring the mechanisms underlying how maternal infection increases the risk for schizophrenia and autism in the offspring. Several mouse models of maternal immune activation (MIA) were used to examine the immediate effects of MIA induced by influenza virus, poly(I:C) and interleukin IL-6 on the fetal brain transcriptome. Our results indicate that all three MIA treatments lead to strong and common gene expression changes in the embryonic brain. Most notably, there is an acute and transient upregulation of the a, b and c crystallin gene family. Furthermore, levels of crystallin gene expression are correlated with the severity of MIA as assessed by placental weight. The overall gene expression changes suggest that the response to MIA is a neuroprotective attempt by the developing brain to counteract environmental stress, but at a cost of disrupting typical neuronal differentiation and axonal growth. We propose that this cascade of events might parallel the mechanisms by which environmental insults contribute to the risk of neurodevelopmental disorders such as schizophrenia and autism. Translational Psychiatry (2012) 2, e98; doi:10.1038/tp.2012.24; published online 3 April 2012 Introduction Young and adult mouse MIA offspring10 also exhibit a cerebellar neuropathology that is commonly found in aut- Maternal infection is a risk factor for schizophrenia and ism.11,12 Furthermore, adult mice born to infected mothers autism. -

A Mutation in the Start Codon of C-Crystallin D Leads to Nuclear Cataracts in the Dahl SS/Jr-Ctr Strain
Mamm Genome (2013) 24:95–104 DOI 10.1007/s00335-013-9447-1 A mutation in the start codon of c-crystallin D leads to nuclear cataracts in the Dahl SS/Jr-Ctr strain Ashley C. Johnson • Jonathan W. Lee • Ashlyn C. Harmon • Zaliya Morris • Xuexiang Wang • Jonathan Fratkin • John P. Rapp • Elise Gomez-Sanchez • Michael R. Garrett Received: 14 November 2012 / Accepted: 17 December 2012 / Published online: 13 February 2013 Ó Springer Science+Business Media New York 2013 Abstract Cataracts are a major cause of blindness. The Ctr 9 SHR) 9 SHR backcross population. The 1-Mbp most common forms of cataracts are age- and UV-related region contained only 13 genes, including 4 genes from the and develop mostly in the elderly, while congenital cataracts c-crystallins (Cryg) gene family, which are known to play a appear at birth or in early childhood. The Dahl salt-sensitive role in cataract formation. All of the c-crystallins were (SS/Jr) rat is an extensively used model of salt-sensitive sequenced and a novel point mutation in the start codon hypertension that exhibits concomitant renal disease. In the (ATG ? GTG) of the Crygd gene was identified. This led to mid-1980s, cataracts appeared in a few animals in the Dahl S the complete absence of the CRYGD protein in the eyes of colony, presumably the result of a spontaneous mutation. the SS/Jr-Ctr strain. In summary, the identification of the The mutation was fixed and bred to establish the SS/Jr-Ctr genetic cause in this novel cataract model may provide an substrain. -

Mouse Models of Congenital Cataract
Mouse models of JOCHEN GRAW congenital cataract Abstract approach to collecting murine cataract mutations was initiated about 20 years ag02 Mouse mutants affecting lens development using paternal treatment of germ cells by X-raT are excellent models for corresponding human and later ethylnitrosourea.3 Among the disorders. The mutant aphakia has been offspring, dominant cataract mutations were characterised by bilaterally aphakic eyes identified by slit lamp observations and (Varnum and Stevens, J Hered 1968;59:147-50); subsequent genetic confirmation. The the corresponding gene was mapped to Neuherberg collection of cataracts contains now chromosome 19 (Varnum and Stevens, Mouse about 150 lines of independent origin and News Lett 1975;53:35). Recent investigations in distinct phenotypes.4,s our laboratory refined the linkage of 0.6 cM proximal to the marker D19Mitl0. Several candidate genes have been excluded (Chukl, Mouse models affecting early eye and lens FgfS, Lbpl, Npm3, Pax2, Pitx3). The Cat3 development mutations are characterised by vacuolated One of the most important genes in eye lenses caused by alterations in the initial development is the paired-box gene Pax6, which secondary lens fibre cell differentiation. is affected in various alleles of the mouse and Secondary malformations develop at the rat Small eye (Sey) mutants.6,7 In homozygous cornea and iris, but the retina remains Sey mice, eyes and nasal cavities do not unaffected. The mutation has been mapped to develop; the animals die soon after birth. The chromosome 10 close to the markers DlOMit41 histological analysis of homozygous mutants and DI0Mit95. Several candidate genes have demonstrated the presence of optic vesicles, but been excluded (Den, Elk3, Lde, MellS, Tr2-11). -

A Novel Nonsense Mutation in CRYGC Is Associated with Autosomal Dominant Congenital Nuclear Cataracts and Microcornea
Molecular Vision 2009; 15:276-282 <http://www.molvis.org/molvis/v15/a28> © 2009 Molecular Vision Received 28 October 2008 | Accepted 29 January 2009 | Published 6 February 2009 A novel nonsense mutation in CRYGC is associated with autosomal dominant congenital nuclear cataracts and microcornea Lu Zhang,1 Songbin Fu,2 Yangshan Ou,1 Tingting Zhao,1 Yunjuan Su,1 Ping Liu1 1Eye hospital, the First Affiliated Hospital, Harbin Medical University, Harbin, China; 2Laboratory of Medical Genetics, Harbin Medical University, Harbin, China Purpose: To report the identification of a novel nonsense mutation in CRYGC in a Chinese family with autosomal dominant congenital nuclear cataracts and microcornea. Methods: We investigated a four-generation Chinese family with six members affected with nuclear cataracts and microcornea. The family resides in a relatively isolated region of northern China. Genomic DNA was isolated from blood leucocytes, genotyping was performed using more than 100 microsatellite markers for the known cataract candidate gene loci, and LOD scores were calculated using the LINKAGE programs. Mutations were detected by DNA sequence analysis of the candidate genes. Results: Evidence for linkage was detected at marker D2S325 (LOD score [Z]=2.29, recombination fraction [θ]=0.0), which closely flanks the γ-crystallin gene cluster (CRYGA-CRYGD) on chromosome 2q32.3-q35. Direct sequencing of the candidate CRYGA-CRYGD gene cluster revealed a c.470G>A transversion in exon 3 of CRYGC, which cosegregated with cataracts in the family and was not observed in 100 normal controls. This single nucleotide change was predicted to introduce a translation stop codon at tryptophan 157 (W157X). -

Downloaded from Public Repository (Massive, MSV000078532) and the Numbers of MS/MS Spectra
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.26.116228; this version posted May 26, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Cataract-associated New Mutants S175G/H181Q of βΒ2-Crystallin and P24S/S31G of γD- Crystallin are Involved in Protein aggregation by Structural Changes In-Kang Song1, Seungjin Na2, Eunok Paek2 Kong-Joo Lee1* 1 Graduate School of Pharmaceutical Sciences and College of Pharmacy, Ewha Womans University, Seoul 03760, South Korea. 2 Department of Computer Science, Hanyang University, Seoul 04763, South Korea *Corresponding author: [email protected] KEYWORDS: βΒ2-Crystallin; γD-Crystallin; cataract-associated mutants; proteomics dataset; hydrogen-deuterium exchange-MS; structural change; stability change; post translational modification; protein aggregation bioRxiv preprint doi: https://doi.org/10.1101/2020.05.26.116228; this version posted May 26, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. ABSTRACT. (250) β/γ-Crystallins, the main structural protein in human lenses, have highly stable structure for keeping the lens transparent. Their mutations have been linked to congenital cataracts. In this study, we identified 10 new mutations of β/γ-crystallins in lens proteomic dataset of cataract patients using bioinformatic tools. Of these, two double mutants, S175G/H181Q of βΒ2-crystallin and P24S/S31G of γD-crystallin, were found mutations occurred in the largest loop linking the distant β-sheets in the Greek key motif.