Biogeochemical and Metabolic Responses to the Flood Pulse in a Semi-Arid Floodplain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(P 117-140) Flood Pulse.Qxp
117 THE FLOOD PULSE CONCEPT: NEW ASPECTS, APPROACHES AND APPLICATIONS - AN UPDATE Junk W.J. Wantzen K.M. Max-Planck-Institute for Limnology, Working Group Tropical Ecology, P.O. Box 165, 24302 Plön, Germany E-mail: [email protected] ABSTRACT The flood pulse concept (FPC), published in 1989, was based on the scientific experience of the authors and published data worldwide. Since then, knowledge on floodplains has increased considerably, creating a large database for testing the predictions of the concept. The FPC has proved to be an integrative approach for studying highly diverse and complex ecological processes in river-floodplain systems; however, the concept has been modified, extended and restricted by several authors. Major advances have been achieved through detailed studies on the effects of hydrology and hydrochemistry, climate, paleoclimate, biogeography, biodi- versity and landscape ecology and also through wetland restoration and sustainable management of flood- plains in different latitudes and continents. Discussions on floodplain ecology and management are greatly influenced by data obtained on flow pulses and connectivity, the Riverine Productivity Model and the Multiple Use Concept. This paper summarizes the predictions of the FPC, evaluates their value in the light of recent data and new concepts and discusses further developments in floodplain theory. 118 The flood pulse concept: New aspects, INTRODUCTION plain, where production and degradation of organic matter also takes place. Rivers and floodplain wetlands are among the most threatened ecosystems. For example, 77 percent These characteristics are reflected for lakes in of the water discharge of the 139 largest river systems the “Seentypenlehre” (Lake typology), elaborated by in North America and Europe is affected by fragmen- Thienemann and Naumann between 1915 and 1935 tation of the river channels by dams and river regula- (e.g. -

Deposition Patterns and Rates of Mining-Contaminated Sediment Within a Sedimentation Basin System, S.E
BearWorks Institutional Repository MSU Graduate Theses Spring 2017 Deposition Patterns and Rates of Mining- Contaminated Sediment within a Sedimentation Basin System, S.E. Missouri Joshua Carl Voss Missouri State University - Springfield, [email protected] Follow this and additional works at: http://bearworks.missouristate.edu/theses Part of the Environmental Indicators and Impact Assessment Commons, Environmental Monitoring Commons, and the Hydrology Commons Recommended Citation Voss, Joshua Carl, "Deposition Patterns and Rates of Mining-Contaminated Sediment within a Sedimentation Basin System, S.E. Missouri" (2017). MSU Graduate Theses. 3074. http://bearworks.missouristate.edu/theses/3074 This article or document was made available through BearWorks, the institutional repository of Missouri State University. The orkw contained in it may be protected by copyright and require permission of the copyright holder for reuse or redistribution. For more information, please contact [email protected]. DEPOSITION PATTERNS AND RATES OF MINING-CONTAMINATED SEDIMENT WITHIN A SEDIMENTATION BASIN SYSTEM, BIG RIVER, S.E. MISSOURI A Masters Thesis Presented to The Graduate College of Missouri State University ATE In Partial Fulfillment Of the Requirements for the Degree Master of Science, Geospatial Sciences in Geography, Geology, and Planning By Josh C. Voss May 2017 Copyright 2017 by Joshua Carl Voss ii DEPOSITION PATTERNS AND RATES OF MINING-CONTAMINATED SEDIMENT WITHIN A SEDIMENTATION BASIN SYSTEM, BIG RIVER, S.E. MISSOURI Geography, Geology, and Planning Missouri State University, May 2017 Master of Science Josh C. Voss ABSTRACT Flooding events exert a dominant control over the deposition and formation of floodplains. The rate at which floodplains form depends on flood magnitude, frequency, and duration, and associated sediment transport capacity and supply. -

Urban Flooding Mitigation Techniques: a Systematic Review and Future Studies
water Review Urban Flooding Mitigation Techniques: A Systematic Review and Future Studies Yinghong Qin 1,2 1 College of Civil Engineering and Architecture, Guilin University of Technology, Guilin 541004, China; [email protected]; Tel.: +86-0771-323-2464 2 College of Civil Engineering and Architecture, Guangxi University, 100 University Road, Nanning 530004, China Received: 20 November 2020; Accepted: 14 December 2020; Published: 20 December 2020 Abstract: Urbanization has replaced natural permeable surfaces with roofs, roads, and other sealed surfaces, which convert rainfall into runoff that finally is carried away by the local sewage system. High intensity rainfall can cause flooding when the city sewer system fails to carry the amounts of runoff offsite. Although projects, such as low-impact development and water-sensitive urban design, have been proposed to retain, detain, infiltrate, harvest, evaporate, transpire, or re-use rainwater on-site, urban flooding is still a serious, unresolved problem. This review sequentially discusses runoff reduction facilities installed above the ground, at the ground surface, and underground. Mainstream techniques include green roofs, non-vegetated roofs, permeable pavements, water-retaining pavements, infiltration trenches, trees, rainwater harvest, rain garden, vegetated filter strip, swale, and soakaways. While these techniques function differently, they share a common characteristic; that is, they can effectively reduce runoff for small rainfalls but lead to overflow in the case of heavy rainfalls. In addition, most of these techniques require sizable land areas for construction. The end of this review highlights the necessity of developing novel, discharge-controllable facilities that can attenuate the peak flow of urban runoff by extending the duration of the runoff discharge. -

Lesson 4: Sediment Deposition and River Structures
LESSON 4: SEDIMENT DEPOSITION AND RIVER STRUCTURES ESSENTIAL QUESTION: What combination of factors both natural and manmade is necessary for healthy river restoration and how does this enhance the sustainability of natural and human communities? GUIDING QUESTION: As rivers age and slow they deposit sediment and form sediment structures, how are sediments and sediment structures important to the river ecosystem? OVERVIEW: The focus of this lesson is the deposition and erosional effects of slow-moving water in low gradient areas. These “mature rivers” with decreasing gradient result in the settling and deposition of sediments and the formation sediment structures. The river’s fast-flowing zone, the thalweg, causes erosion of the river banks forming cliffs called cut-banks. On slower inside turns, sediment is deposited as point-bars. Where the gradient is particularly level, the river will branch into many separate channels that weave in and out, leaving gravel bar islands. Where two meanders meet, the river will straighten, leaving oxbow lakes in the former meander bends. TIME: One class period MATERIALS: . Lesson 4- Sediment Deposition and River Structures.pptx . Lesson 4a- Sediment Deposition and River Structures.pdf . StreamTable.pptx . StreamTable.pdf . Mass Wasting and Flash Floods.pptx . Mass Wasting and Flash Floods.pdf . Stream Table . Sand . Reflection Journal Pages (printable handout) . Vocabulary Notes (printable handout) PROCEDURE: 1. Review Essential Question and introduce Guiding Question. 2. Hand out first Reflection Journal page and have students take a minute to consider and respond to the questions then discuss responses and questions generated. 3. Handout and go over the Vocabulary Notes. Students will define the vocabulary words as they watch the PowerPoint Lesson. -

Seasonal Flooding Affects Habitat and Landscape Dynamics of a Gravel
Seasonal flooding affects habitat and landscape dynamics of a gravel-bed river floodplain Katelyn P. Driscoll1,2,5 and F. Richard Hauer1,3,4,6 1Systems Ecology Graduate Program, University of Montana, Missoula, Montana 59812 USA 2Rocky Mountain Research Station, Albuquerque, New Mexico 87102 USA 3Flathead Lake Biological Station, University of Montana, Polson, Montana 59806 USA 4Montana Institute on Ecosystems, University of Montana, Missoula, Montana 59812 USA Abstract: Floodplains are comprised of aquatic and terrestrial habitats that are reshaped frequently by hydrologic processes that operate at multiple spatial and temporal scales. It is well established that hydrologic and geomorphic dynamics are the primary drivers of habitat change in river floodplains over extended time periods. However, the effect of fluctuating discharge on floodplain habitat structure during seasonal flooding is less well understood. We collected ultra-high resolution digital multispectral imagery of a gravel-bed river floodplain in western Montana on 6 dates during a typical seasonal flood pulse and used it to quantify changes in habitat abundance and diversity as- sociated with annual flooding. We observed significant changes in areal abundance of many habitat types, such as riffles, runs, shallow shorelines, and overbank flow. However, the relative abundance of some habitats, such as back- waters, springbrooks, pools, and ponds, changed very little. We also examined habitat transition patterns through- out the flood pulse. Few habitat transitions occurred in the main channel, which was dominated by riffle and run habitat. In contrast, in the near-channel, scoured habitats of the floodplain were dominated by cobble bars at low flows but transitioned to isolated flood channels at moderate discharge. -

Modifying Wepp to Improve Streamflow Simulation in a Pacific Northwest Watershed
MODIFYING WEPP TO IMPROVE STREAMFLOW SIMULATION IN A PACIFIC NORTHWEST WATERSHED A. Srivastava, M. Dobre, J. Q. Wu, W. J. Elliot, E. A. Bruner, S. Dun, E. S. Brooks, I. S. Miller ABSTRACT. The assessment of water yield from hillslopes into streams is critical in managing water supply and aquatic habitat. Streamflow is typically composed of surface runoff, subsurface lateral flow, and groundwater baseflow; baseflow sustains the stream during the dry season. The Water Erosion Prediction Project (WEPP) model simulates surface runoff, subsurface lateral flow, soil water, and deep percolation. However, to adequately simulate hydrologic conditions with significant quantities of groundwater flow into streams, a baseflow component for WEPP is needed. The objectives of this study were (1) to simulate streamflow in the Priest River Experimental Forest in the U.S. Pacific Northwest using the WEPP model and a baseflow routine, and (2) to compare the performance of the WEPP model with and without including the baseflow using observed streamflow data. The baseflow was determined using a linear reservoir model. The WEPP- simulated and observed streamflows were in reasonable agreement when baseflow was considered, with an overall Nash- Sutcliffe efficiency (NSE) of 0.67 and deviation of runoff volume (Dv) of 7%. In contrast, the WEPP simulations without including baseflow resulted in an overall NSE of 0.57 and Dv of 47%. On average, the simulated baseflow accounted for 43% of the streamflow and 12% of precipitation annually. Integration of WEPP with a baseflow routine improved the model’s applicability to watersheds where groundwater contributes to streamflow. Keywords. Baseflow, Deep seepage, Forest watershed, Hydrologic modeling, Subsurface lateral flow, Surface runoff, WEPP. -

Comparison of Two Methods for Estimating Base Flow in Selected Reaches of the South Platte River, Colorado
Prepared in cooperation with the Colorado Water Conservation Board Comparison of Two Methods for Estimating Base Flow in Selected Reaches of the South Platte River, Colorado Scientific Investigations Report 2012–5034 U.S. Department of the Interior U.S. Geological Survey Comparison of Two Methods for Estimating Base Flow in Selected Reaches of the South Platte River, Colorado By Joseph P. Capesius and L. Rick Arnold Prepared in cooperation with the Colorado Water Conservation Board Scientific Investigations Report 2012–5034 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this report is in the public domain, permission must be secured from the individual copyright owners to reproduce any copyrighted materials contained within this report. Suggested citation: Capesius, J.P., and Arnold, L.R., 2012, Comparison of two methods for estimating base flow in selected reaches of the South Platte River, Colorado: U.S. Geological Survey Scientific Investigations Report 2012–5034, 20 p. -

Variable Hydrologic and Geomorphic Responses to Intentional Levee Breaches Along the Lower Cosumnes River, California
Received: 21 April 2016 Revised: 29 March 2017 Accepted: 30 March 2017 DOI: 10.1002/rra.3159 RESEARCH ARTICLE Not all breaks are equal: Variable hydrologic and geomorphic responses to intentional levee breaches along the lower Cosumnes River, California A. L. Nichols1 | J. H. Viers1,2 1 Center for Watershed Sciences, University of California, Davis, California, USA Abstract 2 School of Engineering, University of The transport of water and sediment from rivers to adjacent floodplains helps generate complex California, Merced, California, USA floodplain, wetland, and riparian ecosystems. However, riverside levees restrict lateral connectiv- Correspondence ity of water and sediment during flood pulses, making the re‐introduction of floodplain hydrogeo- A. L. Nichols, Center for Watershed Sciences, morphic processes through intentional levee breaching and removal an emerging floodplain University of California, Davis, California, USA. restoration practice. Repeated topographic observations from levee breach sites along the lower Email: [email protected] Cosumnes River (USA) indicated that breach architecture influences floodplain and channel hydrogeomorphic processes. Where narrow breaches (<75 m) open onto graded floodplains, Funding information California Department of Fish and Wildlife archetypal crevasse splays developed along a single dominant flowpath, with floodplain erosion (CDFW) Ecosystem Restoration Program in near‐bank areas and lobate splay deposition in distal floodplain regions. Narrow breaches (ERP), Grant/Award Number: E1120001; The opening into excavated floodplain channels promoted both transverse advection and turbulent Nature Concervancy (TNC); Consumnes River Preserve diffusion of sediment into the floodplain channel, facilitating near‐bank deposition and potential breach closure. Wide breaches (>250 m) enabled multiple modes of water and sediment transport onto graded floodplains. -

Isotopes \&Amp\; Geochemistry: Tools for Geothermal Reservoir
E3S Web of Conferences 98, 08013 (2019) https://doi.org/10.1051/e3sconf/20199808013 WRI-16 Isotopes & Geochemistry: Tools For Geothermal Reservoir Characterization (Kamchatka Examples) Alexey Kiryukhin1,*, Pavel Voronin1, Nikita Zhuravlev1, Andrey Polyakov1, Tatiana Rychkova1, Vasily Lavrushin2, Elena Kartasheva1, Natalia Asaulova3, Larisa Vorozheikina3, and Ivan Chernev4 1Institute of Volcanology & Seismology FEB RAS, Piip 9, Petropavlovsk-Kamch., 683006, Russia 2Geological Institute RAS, Pyzhevsky 7, Moscow 119017, Russia 3Teplo Zemli JSC, Vilyuchinskaya 6, Thermalny, 684000, Russia 4Geotherm JSC, Ac. Koroleva 60, Petropavlovsk-Kamchatsky, 683980, Russia Abstract. The thermal, hydrogeological, and chemical processes affecting Kamchatka geothermal reservoirs were studied by using isotope and geochemistry data: (1) The Geysers Valley hydrothermal reservoirs; (2) The Paratunsky low temperature reservoirs; (3) The North-Koryaksky hydrothermal system; (4) The Mutnovsky high temperature geothermal reservoir; (5) The Pauzhetsky geothermal reservoir. In most cases water isotope in combination with Cl- transient data are found to be useful tool to estimate reservoirs natural and disturbed by exploitation recharge conditions, isotopes of carbon-13 (in CO2) data are pointed either active magmatic recharge took place, while SiO2 and Na-K geothermometers shows opposite time transient trends (Paratunsky, Geysers Valley) suggest that it is necessary to use more complicated geochemical systems of water/mineral equilibria. 1 Introduction Active pore space limitation mass transport velocities are typically greater than heat transport velocities. That is a fundamental reason why changes in the isotope and chemistry parameters of geofluids are faster than changes in the heat properties of producing geothermal reservoirs, which makes them pre-cursors to production parameter changes. In cases of inactive to rock chemical species, they can be used as tracers of fluid flow and boundary condition estimation. -

Estimation of the Base Flow Recession Constant Under Human Interference Brian F
WATER RESOURCES RESEARCH, VOL. 49, 7366–7379, doi:10.1002/wrcr.20532, 2013 Estimation of the base flow recession constant under human interference Brian F. Thomas,1 Richard M. Vogel,2 Charles N. Kroll,3 and James S. Famiglietti1,4,5 Received 28 January 2013; revised 27 August 2013; accepted 13 September 2013; published 15 November 2013. [1] The base flow recession constant, Kb, is used to characterize the interaction of groundwater and surface water systems. Estimation of Kb is critical in many studies including rainfall-runoff modeling, estimation of low flow statistics at ungaged locations, and base flow separation methods. The performance of several estimators of Kb are compared, including several new approaches which account for the impact of human withdrawals. A traditional semilog estimation approach adapted to incorporate the influence of human withdrawals was preferred over other derivative-based estimators. Human withdrawals are shown to have a significant impact on the estimation of base flow recessions, even when withdrawals are relatively small. Regional regression models are developed to relate seasonal estimates of Kb to physical, climatic, and anthropogenic characteristics of stream-aquifer systems. Among the factors considered for explaining the behavior of Kb, both drainage density and human withdrawals have significant and similar explanatory power. We document the importance of incorporating human withdrawals into models of the base flow recession response of a watershed and the systemic downward bias associated with estimates of Kb obtained without consideration of human withdrawals. Citation: Thomas, B. F., R. M. Vogel, C. N. Kroll, and J. S. Famiglietti (2013), Estimation of the base flow recession constant under human interference, Water Resour. -

Classifying Rivers - Three Stages of River Development
Classifying Rivers - Three Stages of River Development River Characteristics - Sediment Transport - River Velocity - Terminology The illustrations below represent the 3 general classifications into which rivers are placed according to specific characteristics. These categories are: Youthful, Mature and Old Age. A Rejuvenated River, one with a gradient that is raised by the earth's movement, can be an old age river that returns to a Youthful State, and which repeats the cycle of stages once again. A brief overview of each stage of river development begins after the images. A list of pertinent vocabulary appears at the bottom of this document. You may wish to consult it so that you will be aware of terminology used in the descriptive text that follows. Characteristics found in the 3 Stages of River Development: L. Immoor 2006 Geoteach.com 1 Youthful River: Perhaps the most dynamic of all rivers is a Youthful River. Rafters seeking an exciting ride will surely gravitate towards a young river for their recreational thrills. Characteristically youthful rivers are found at higher elevations, in mountainous areas, where the slope of the land is steeper. Water that flows over such a landscape will flow very fast. Youthful rivers can be a tributary of a larger and older river, hundreds of miles away and, in fact, they may be close to the headwaters (the beginning) of that larger river. Upon observation of a Youthful River, here is what one might see: 1. The river flowing down a steep gradient (slope). 2. The channel is deeper than it is wide and V-shaped due to downcutting rather than lateral (side-to-side) erosion. -
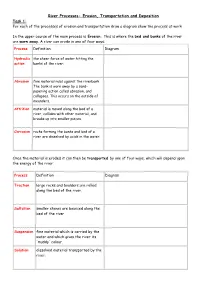
River Processes- Erosion, Transportation and Deposition Task 1: for Each of the Processes of Erosion and Transportation Draw a Diagram Show the Process at Work
River Processes- Erosion, Transportation and Deposition Task 1: For each of the processes of erosion and transportation draw a diagram show the process at work In the upper course of the main process is Erosion. This is where the bed and banks of the river are worn away. A river can erode in one of four ways: Process Definition Diagram Hydraulic the sheer force of water hitting the action banks of the river: Abrasion fine material rubs against the riverbank The bank is worn away by a sand- papering action called abrasion, and collapses. This occurs on the outside of meanders. Attrition material is moved along the bed of a river, collides with other material, and breaks up into smaller pieces. Corrosion rocks forming the banks and bed of a river are dissolved by acids in the water. Once the material is eroded it can then be transported by one of four ways, which will depend upon the energy of the river: Process Definition Diagram Traction large rocks and boulders are rolled along the bed of the river. Saltation smaller stones are bounced along the bed of the river Suspension fine material which is carried by the water and which gives the river its 'muddy' colour. Solution dissolved material transported by the river. In the middle and lower course, the land is much flatter, this means that the river is flowing more slowly and has much less energy. The river starts to deposit (drop) the material that it has been carry Deposition Challenge: Add labels onto the diagram to show where all of the processes could be happening in the river channel.