The Evolution of Body Size in the Order Siluriformes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Vampire Fish Rides Giant Catfishes in The
FREE MEALS ON LONG-DISTANCE CRUISERS: THE VAMPIRE FISH RIDES GIANT CATFISHES IN THE AMAZON Jansen Zuanon*, Ivan Sazima** Biota Neotropica v5 (n1) – http://www.biotaneotropica.org.br/v5n1/pt/abstract?article+BN03005012005 Recebido em 16/08/04 - Versão revisada recebida em 21/01/05 - Publicado em 10/02/05. *CPBA, Caixa Postal 478, INPA-Instituto Nacional de Pesquisas da Amazônia,69083-970 Manaus, Amazonas, Brasil **Departamento de Zoologia e Museu de História Natural, Caixa Postal 6109, Universidade Estadual de Campinas, 13083-970 Campinas, São Paulo, Brazil (www.unicamp.br) **Corresponding author. Tel: + 55-19-3788 7292; fax: +55-19-3289 3124; e-mail: [email protected] Abstract - The trichomycterid catfishes known as candirus are renowned for their blood feeding, but information on their habits under natural conditions is very fragmentary and generally restricted to hosts or habitats. We recorded an undescribed species of the vandelliine genus Paracanthopoma riding the giant jau catfish, Zungaro zungaro (Pimelodidae), in the upper Amazon. The candirus were found on the host’s caudal and pectoral fins, as well as the base of the dorsal fin, with their snouts buried up to the eyes in the tough skin of the catfish host. All of them had small amounts of partly digested blood in the distal part of the gut. Along the host’s dorsal fin base we found a few additional tiny holes, most of them healed. We suggest that Paracanthopoma feeds on the gill chamber of its hosts, and that the individuals we found were taking a ride partly buried into the host’s skin. -

Water Diversion in Brazil Threatens Biodiversit
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/332470352 Water diversion in Brazil threatens biodiversity Article in AMBIO A Journal of the Human Environment · April 2019 DOI: 10.1007/s13280-019-01189-8 CITATIONS READS 0 992 12 authors, including: Vanessa Daga Valter Monteiro de Azevedo-Santos Universidade Federal do Paraná 34 PUBLICATIONS 374 CITATIONS 17 PUBLICATIONS 248 CITATIONS SEE PROFILE SEE PROFILE Fernando Pelicice Philip Fearnside Universidade Federal de Tocantins Instituto Nacional de Pesquisas da Amazônia 68 PUBLICATIONS 2,890 CITATIONS 612 PUBLICATIONS 20,906 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Freshwater microscrustaceans from continental Ecuador and Galápagos Islands: Integrative taxonomy and ecology View project Conservation policy View project All content following this page was uploaded by Philip Fearnside on 11 May 2019. The user has requested enhancement of the downloaded file. The text that follows is a PREPRINT. O texto que segue é um PREPRINT. Please cite as: Favor citar como: Daga, Vanessa S.; Valter M. Azevedo- Santos, Fernando M. Pelicice, Philip M. Fearnside, Gilmar Perbiche-Neves, Lucas R. P. Paschoal, Daniel C. Cavallari, José Erickson, Ana M. C. Ruocco, Igor Oliveira, André A. Padial & Jean R. S. Vitule. 2019. Water diversion in Brazil threatens biodiversity: Potential problems and alternatives. Ambio https://doi.org/10.1007/s13280-019- 01189-8 . (online version published 27 April 2019) ISSN: 0044-7447 (print version) ISSN: 1654-7209 (electronic version) Copyright: Royal Swedish Academy of Sciences & Springer Science+Business Media B.V. -

(Niño 3.4 Index) in the West of Java Sea
Available online at www.worldscientificnews.com WSN 117 (2019) 175-182 EISSN 2392-2192 SHORT COMMUNICATION Variability in fish catch rates associated with Sea Surface Temperature Anomaly (Niño 3.4 index) in the West of Java Sea Mega Syamsuddin*, Sunarto, Lintang Yuliadi, Syawaludin Harahap Department of Marine Science, Faculty of Fisheries and Marine Science, Universitas Padjadjaran, Jalan Raya Bandung-Sumedang KM 21 Jatinangor Sumedang Indonesia *E-mail address: [email protected] ABSTRACT The remotely derived oceanographic variables included sea surface height anomaly (SSHA), sea surface temperature (SST), chlorophyll-a (Chl a) and fish catches are used as a combined dataset to understand the ocean climate variability and further addresses their relations with the fish catches in the West-Java Sea. Fish catches and remotely sensed data are analyzed for the 5 years datasets from 2010- 2014 and emphasized the differences of climate conditions during El Niño Southern Oscillation (ENSO) events. Here, we demonstrate the prominent annual variation of two species small pelagic fish catches (Euthynus affinis and Scomberomorus commerson) and one demersal species (Netuma thallasina) as representative of dominant catch in the region. Small pelagic catches had significant increment during El Niño compared to during La Niña events. Changes in oceanographic conditions during ENSO events resulted in perceivable variations in catches, with an average catches of 839.6 t (E. affinis) and 273,7 t (S. commerson) during El Niño. During La Niña event catch rates were reduced with an average catches of 602.6 t (E. affinis) and 210.3 t (S. commerson). During the La Niña event was less favorable for small pelagic catches. -

A New Black Baryancistrus with Blue Sheen from the Upper Orinoco (Siluriformes: Loricariidae)
Copeia 2009, No. 1, 50–56 A New Black Baryancistrus with Blue Sheen from the Upper Orinoco (Siluriformes: Loricariidae) Nathan K. Lujan1, Mariangeles Arce2, and Jonathan W. Armbruster1 Baryancistrus beggini, new species, is described from the upper Rı´o Orinoco and lower portions of its tributaries, the Rı´o Guaviare in Colombia and Rı´o Ventuari in Venezuela. Baryancistrus beggini is unique within Hypostominae in having a uniformly dark black to brown base color with a blue sheen in life, and the first three to five plates of the midventral series strongly bent, forming a distinctive keel above the pectoral fins along each side of the body. It is further distinguished by having a naked abdomen, two to three symmetrical and ordered predorsal plate rows including the nuchal plate, and the last dorsal-fin ray adnate with adipose fin via a posterior membrane that extends beyond the preadipose plate up to half the length of the adipose-fin spine. Se describe una nueva especie, Baryancistrus beggini, del alto Rı´o Orinoco y las partes bajas de sus afluentes: el rı´o Guaviare en Colombia, y el rı´o Ventuari en Venezuela. Baryancistrus beggini es la u´ nica especie entre los Hypostominae que presenta fondo negro oscuro a marro´ n sin marcas, con brillo azuloso en ejemplares vivos. Las primeras tres a cinco placas de la serie medioventral esta´n fuertemente dobladas, formando una quilla notable por encima de las aletas pectorales en cada lado del cuerpo. Baryancistrus beggini se distingue tambie´n por tener el abdomen desnudo, dos o tres hileras de placas predorsales sime´tricas y ordenadas (incluyendo la placa nucal) y el u´ ltimo radio de la aleta dorsal adherido a la adiposa a trave´s de una membrana que se extiende posteriormente, sobrepasando la placa preadiposa y llegando hasta la mitad de la espina adiposa. -

Stock Enhancement Activities in the Union of Myanmar
Stock enhancement activities in the Union of Myanmar Item Type book_section Authors Win, Kyaw Myo Publisher Aquaculture Department, Southeast Asian Fisheries Development Center Download date 25/09/2021 20:31:29 Link to Item http://hdl.handle.net/1834/40487 Stock enhancement activities in the Union of Myanmar Win, Kyaw Myo Date published: 2006 To cite this document : Win, K. M. (2006). Stock enhancement activities in the Union of Myanmar. In J. H. Primavera, E. T. Quinitio, & M. R. R. Eguia (Eds.), Proceedings of the Regional Technical Consultation on Stock Enhancement for Threatened Species of International Concern, Iloilo City, Philippines, 13-15 July 2005 (pp. 117-120). Tigbauan, Iloilo, Philippines: Aquaculture Department, Southeast Asian Fisheries Development Center. To link to this document : http://hdl.handle.net/10862/2940 Share on : PLEASE SCROLL DOWN TO SEE THE FULL TEXT This content was downloaded from SEAFDEC/AQD Institutional Repository (SAIR) - the official digital repository of scholarly and research information of the department Downloaded by: [Anonymous] On: January 30, 2019 at 11:14 PM CST Follow us on: Facebook | Twitter | Google Plus | Instagram Library & Data Banking Services Section | Training & Information Division Aquaculture Department | Southeast Asian Fisheries Development Center (SEAFDEC) Tigbauan, Iloilo 5021 Philippines | Tel: (63-33) 330 7088, (63-33) 330 7000 loc 1340 | Fax: (63-33) 330 7088 Website: www.seafdec.org.ph | Email: [email protected] Copyright © 2011-2015 SEAFDEC Aquaculture Department. Stock Enhancement Activities in the Union of Myanmar Kyaw Myo Win Department of Fisheries, Sinmin Road Ahlone Township, Yangon, Union of Myanmar Introduction Exploitation of endangered species is strictly prohibited hence, there are no The geography of the Union of Myanmar recorded catches for these species. -

Multilocus Molecular Phylogeny of the Suckermouth Armored Catfishes
Molecular Phylogenetics and Evolution xxx (2014) xxx–xxx Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae ⇑ Nathan K. Lujan a,b, , Jonathan W. Armbruster c, Nathan R. Lovejoy d, Hernán López-Fernández a,b a Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, Ontario M5S 2C6, Canada b Department of Ecology and Evolutionary Biology, University of Toronto, Toronto, Ontario M5S 3B2, Canada c Department of Biological Sciences, Auburn University, Auburn, AL 36849, USA d Department of Biological Sciences, University of Toronto Scarborough, Toronto, Ontario M1C 1A4, Canada article info abstract Article history: The Neotropical catfish family Loricariidae is the fifth most species-rich vertebrate family on Earth, with Received 4 July 2014 over 800 valid species. The Hypostominae is its most species-rich, geographically widespread, and eco- Revised 15 August 2014 morphologically diverse subfamily. Here, we provide a comprehensive molecular phylogenetic reap- Accepted 20 August 2014 praisal of genus-level relationships in the Hypostominae based on our sequencing and analysis of two Available online xxxx mitochondrial and three nuclear loci (4293 bp total). Our most striking large-scale systematic discovery was that the tribe Hypostomini, which has traditionally been recognized as sister to tribe Ancistrini based Keywords: on morphological data, was nested within Ancistrini. This required recognition of seven additional tribe- Neotropics level clades: the Chaetostoma Clade, the Pseudancistrus Clade, the Lithoxus Clade, the ‘Pseudancistrus’ Guiana Shield Andes Mountains Clade, the Acanthicus Clade, the Hemiancistrus Clade, and the Peckoltia Clade. -

Permen-Kp/… Tentang Larangan Pemasukan Jenis Ikan Membahayakan Dan Merugikan Ke Dalam Wilayah Negara Republik Indonesia
1 RANCANGAN PERATURAN MENTERI KELAUTAN DAN PERIKANAN REPUBLIK INDONESIA NOMOR …/PERMEN-KP/… TENTANG LARANGAN PEMASUKAN JENIS IKAN MEMBAHAYAKAN DAN MERUGIKAN KE DALAM WILAYAH NEGARA REPUBLIK INDONESIA DENGAN RAHMAT TUHAN YANG MAHA ESA MENTERI KELAUTAN DAN PERIKANAN REPUBLIK INDONESIA, Menimbang : a. bahwa untuk melestarikan sumber daya ikan dan lingkungannya serta untuk mencegah terjadinya hal- hal yang membahayakan dan merugikan masyarakat, pembudidayaan ikan, sumber daya ikan, dan/atau lingkungan sumber daya ikan, perlu mengganti Peraturan Menteri Kelautan dan Perikanan Nomor 41/PERMEN-KP/2014 tentang Larangan Pemasukan Jenis Ikan Berbahaya dari Luar Negeri ke Dalam Wilayah Negara Republik Indonesia; b. bahwa berdasarkan pertimbangan sebagaimana dimaksud dalam huruf a, perlu menetapkan Peraturan Menteri Kelautan dan Perikanan tentang Larangan Pemasukan Jenis Ikan Membahayakan dan Merugikan ke Dalam Wilayah Negara Republik Indonesia; Mengingat : 1. Pasal 17 ayat (3) Undang-Undang Dasar Negara Republik Indonesia Tahun 1945; - 2 - 2. Undang-Undang Nomor 31 Tahun 2004 tentang Perikanan (Lembaran Negara Republik Indonesia Tahun 2004 Nomor 118, Tambahan Lembaran Negara Republik Indonesia Nomor 4433) sebagaimana telah diubah dengan Undang-Undang Nomor 45 Tahun 2009 tentang Perubahan atas Undang-Undang Nomor 31 Tahun 2004 tentang Perikanan (Lembaran Negara Republik Indonesia Tahun 2009 Nomor 154, Tambahan Lembaran Negara Republik Indonesia Nomor 5073); 3. Peraturan Presiden Nomor 63 Tahun 2015 tentang Kementerian Kelautan dan Perikanan (Lembaran Negara Republik Indonesia Tahun 2015 Nomor 111) sebagaimana telah diubah dengan Peraturan Presiden Nomor 2 Tahun 2017 tentang Perubahan atas Peraturan Presiden Nomor 63 Tahun 2015 tentang Kementerian Kelautan dan Perikanan (Lembaran Negara Republik Indonesia Tahun 2017 Nomor 5); 4. Peraturan Presiden Nomor 68 Tahun 2019 tentang Organisasi Kementerian Negara (Lembaran Negara Republik Indonesia Tahun 2019 Nomor 203); 5. -

Global Catfish Biodiversity 17
American Fisheries Society Symposium 77:15–37, 2011 © 2011 by the American Fisheries Society Global Catfi sh Biodiversity JONATHAN W. ARMBRUSTER* Department of Biological Sciences, Auburn University 331 Funchess, Auburn University, Alabama 36849, USA Abstract.—Catfi shes are a broadly distributed order of freshwater fi shes with 3,407 cur- rently valid species. In this paper, I review the different clades of catfi shes, all catfi sh fami- lies, and provide information on some of the more interesting aspects of catfi sh biology that express the great diversity that is present in the order. I also discuss the results of the widely successful All Catfi sh Species Inventory Project. Introduction proximately 10.8% of all fi shes and 5.5% of all ver- tebrates are catfi shes. Renowned herpetologist and ecologist Archie Carr’s But would every one be able to identify the 1941 parody of dichotomous keys, A Subjective Key loricariid catfi sh Pseudancistrus pectegenitor as a to the Fishes of Alachua County, Florida, begins catfi sh (Figure 2A)? It does not have scales, but it with “Any damn fool knows a catfi sh.” Carr is right does have bony plates. It is very fl at, and its mouth but only in part. Catfi shes (the Siluriformes) occur has long jaws but could not be called large. There is on every continent (even fossils are known from a barbel, but you might not recognize it as one as it Antarctica; Figure 1); and the order is extremely is just a small extension of the lip. There are spines well supported by numerous complex synapomor- at the front of the dorsal and pectoral fi ns, but they phies (shared, derived characteristics; Fink and are not sharp like in the typical catfi sh. -

Siluriformes: Loricariidae) from the Western Guiana Shield
Copeia 2011, No. 2, 216–225 Two New Genera and Species of Ancistrini (Siluriformes: Loricariidae) from the Western Guiana Shield Nathan K. Lujan1,2 and Jonathan W. Armbruster1 Two new ancistrin genera and species are described from main channels and tributaries of the upper Orinoco River in Amazonas, Venezuela. Micracanthicus vandragti is black with white spots and distinguished by its small body-size, large gas-bladder capsules, and highly protrusible mandibles with short tooth cups and five to eight long teeth per tooth cup. The known range of Micracanthicus vandragti is restricted to the lower Ventuari River and portions of the Orinoco River near its rocky, anastomose confluence with the Ventuari River. Soromonichthys stearleyi is green with small yellow-gold spots on the head and thin vertical bars on the body and has long dentary and premaxillary tooth cups with 39–69 teeth per tooth cup. It is distinguished by its coloration and by its unique pattern of plate loss on the snout: plates absent from mesethmoid surface and anteriormost margin of snout. Soromonichthys stearleyi is known only from Soromoni Creek, a northern tributary of the upper Orinoco draining southern slopes of Mount Duida. NGOING ichthyological inventories of the upper process of Weberian complex perforated distally with large Orinoco Basin in Amazonas, Venezuela, by the first foramina (vs. not or only mildly perforated distally); O author and colleagues have yielded many new and branched anal-fin rays five (vs. four); coracoid posterior interesting suckermouth armored catfishes (Loricariidae; process elongated, thin, pointed (vs. distal end about as wide Lujan et al., 2007, 2009; Lujan, 2008). -
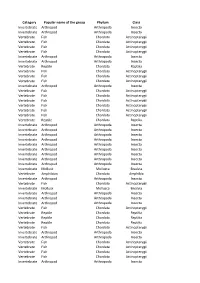
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Making a Big Splash with Louisiana Fishes
Making a Big Splash with Louisiana Fishes Written and Designed by Prosanta Chakrabarty, Ph.D., Sophie Warny, Ph.D., and Valerie Derouen LSU Museum of Natural Science To those young people still discovering their love of nature... Note to parents, teachers, instructors, activity coordinators and to all the fishermen in us: This book is a companion piece to Making a Big Splash with Louisiana Fishes, an exhibit at Louisiana State Universi- ty’s Museum of Natural Science (MNS). Located in Foster Hall on the main campus of LSU, this exhibit created in 2012 contains many of the elements discussed in this book. The MNS exhibit hall is open weekdays, from 8 am to 4 pm, when the LSU campus is open. The MNS visits are free of charge, but call our main office at 225-578-2855 to schedule a visit if your group includes 10 or more students. Of course the book can also be enjoyed on its own and we hope that you will enjoy it on your own or with your children or students. The book and exhibit was funded by the Louisiana Board Of Regents, Traditional Enhancement Grant - Education: Mak- ing a Big Splash with Louisiana Fishes: A Three-tiered Education Program and Museum Exhibit. Funding was obtained by LSUMNS Curators’ Sophie Warny and Prosanta Chakrabarty who designed the exhibit with Southwest Museum Services who built it in 2012. The oarfish in the exhibit was created by Carolyn Thome of the Smithsonian, and images exhibited here are from Curator Chakrabarty unless noted elsewhere (see Appendix II).