Survey on Physico-Chemical Investigation and Identification of Toxic Compounds in Mandideep Industrial Area
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Construction of 6-Lane Bhopal – Indore Green Field Expressway – 146.880 Kms in the State of Madhya Pradesh Alternative Option Analysis- Annexure-III
Construction of 6-Lane Bhopal – Indore Green Field Expressway – 146.880 Kms in the State of Madhya Pradesh Alternative Option Analysis- Annexure-III Criteria for Fixing Alignment for Expressways 1. The Expressway between two terminal stations should be short and straight as far as possible, but due to engineering, social and environmental considerations some deviations may be required. 2. The project should be constructible and easy to maintain; the Greenfield project should reduce the vehicle operation cost with respect to the existing option already available i.e. using the NH/SHs in combination to reach from point A to point B. 3. It should be safe at all stages i.e. during design, construction and operation stages. Safety audits at each stage should confirm the same. 4. The project initial cost, maintenance cost, and operating cost should be optimum so as to be considered economical with respect to its options. 5. The alignment should be finalised giving due consideration to sitting/location of major structures including Major/Minor Bridges, Interchanges and ROBs. The space requirement of interchanges to be kept into consideration to avoid major resettlement. 6. Tunnel / Box cutting of Hills should be considered as the last option and should be provided only when it is absolutely necessary. 7. The location of spurs for connecting the important towns to be decided while fixing the alignment Options. 8. The alignment should follow the unused / barren land to the extent possible to reduce the cost of land acquisition. 9. The proposed options in the present case connects the under developed regions of Madhya Pradesh which would lead to the development of new growth centres along the proposed highway i.e. -

Nagar Parishad, Rehti District - Sehore (M.P.) Map Title
77°24'40"E 77°25'0"E 77°25'20"E 77°25'40"E 77°26'0"E 77°26'20"E 77°26'40"E 77°27'0"E Nagar Parishad, Rehti District - Sehore (M.P.) Map Title ! ! j ! ! CITY BASE MAP ! n N ! N ! " ! a " ! ! 0 0 ! ! g ! 2 2 ! ! ' a ' ! l ! ! 5 l 5 ! ! ! 4 ! u 4 ! ° ° ! ! ! 2 d 2 ! ! ! i ! 2 2 ! ! a ! ! ! ! b Legend ! ! ! ! ! O ! ! ! ! ! ! % ! o ! ! ! T ! ! ! ! ! ! ! ! ! ! ! ! ! Important Landmarks ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Municipal Area Boundary ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !! ! ! ! ! ! ! ! t ! ! ! c ! ! a ! ! Railway Line ! ! y ! ! z ! ! r a ! ! ! e ! B ! ! v ! ! a i ! i ! l ! ! ! R a ! ! ! p ! r ! i National Highway ! ! a ! P ! ! ! b ! o ! ! ! T b # ! ! ! a ! Tehsil ! ! ! B ! ! ! ! ! State Highway ! o ! ! ! ! ! ! ! ! ! ! T ! ! ! ! ! ! ! ! N ! N ! ! " ! ! " ! ! ! ! ! 0 0 ! ! ' ! ! ! ' ! ! ! ! ! 5 5 ! ! ! ! ! ! ! 4 ! 4 ! ! ! ! ! ° ! ° ! ! ! ! ! ! 2 2 ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 2 ! 2 Major Road ! ! ! ! ! ! ! ! ! ! ! # ! ! ! ! ! ! ! ! Aara Machine ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! 2 ! 2 ! ! - ! ! ! Other Road H S ! ! ! ! ! ! ! j ! ! ! ! ! ! ! ! ! ! River se # ! u Ware Ho ! ! ! ! ! ! ! ! ! j ! ! ! # ! ! ! ! Balbir Singh House ! ! ! ! ! Drainage / Nala ! ! ! ! # ! ! ! Maulana Steel Fabrication ! ! Ay j # odhya Bas ! ! ti ! ! ! ! ! ! ! ! ! ! ! Canal ! ! ! ! # ! ! Salma Bee ! ! r ! ! e ! ! v ! i d oa ! R ! R ! r ya ! i ! a ad ! r ! b Bu Holkar Singh # Pond / Tank / Reservoir Ho ! b i use ! a nd ! u ! N B Ga N ! " " ! ! 0 0 ! ! 4 4 ' ' ! ! 4 4 ! ! 4 4 ! ! ° ° ! 2 2 ! ! 2 2 # ! ! ! ! -

Bank Wise-District Wise Bank Branches (Excluding Cooperative
Bank wise-District wise Bank Branches (Excluding Cooperative Bank/District No. of Branches Allahabad Bank 205 Agar-Malwa 2 Anuppur 2 Balaghat 4 Bhopal 25 Burhanpur 1 Chhatarpur 3 Chhindwara 8 Damoh 3 Datia 1 Dewas 1 Dhar 1 Dindori 1 East Nimar 1 Gwalior 3 Harda 1 Hoshangabad 3 Indore 12 Jabalpur 24 Katni 6 Mandla 4 Mandsaur 2 Morena 1 Narsinghpur 7 Neemuch 2 Panna 3 Raisen 1 Rajgarh 2 Ratlam 2 Rewa 16 Sagar 6 Satna 28 Sehore 2 Seoni 2 Shahdol 3 Shajapur 1 Shivpuri 2 Sidhi 5 Singrauli 6 Tikamgarh 1 Ujjain 2 Vidisha 4 West Nimar 1 Andhra Bank 45 Betul 1 Bhind 1 Bhopal 8 Burhanpur 1 Chhindwara 1 Dewas 1 Dhar 1 East Nimar 1 Gwalior 2 Harda 1 Hoshangabad 2 Indore 11 Jabalpur 3 Katni 1 Narsinghpur 2 Rewa 1 Sagar 1 Satna 1 Sehore 2 Ujjain 1 Vidisha 2 Au Small Finance Bank Ltd. 37 Agar-Malwa 1 Barwani 1 Betul 1 Bhopal 2 Chhatarpur 1 Chhindwara 2 Dewas 2 Dhar 2 East Nimar 1 Hoshangabad 1 Indore 2 Jabalpur 1 Katni 1 Mandla 1 Mandsaur 2 Neemuch 1 Raisen 2 Rajgarh 1 Ratlam 2 Rewa 1 Satna 1 Sehore 2 Shajapur 1 Tikamgarh 1 Ujjain 1 Vidisha 2 West Nimar 1 Axis Bank Ltd. 136 Agar-Malwa 1 Alirajpur 1 Anuppur 1 Ashoknagar 1 Balaghat 1 Barwani 3 Betul 2 Bhind 1 Bhopal 20 Burhanpur 1 Chhatarpur 1 Chhindwara 2 Damoh 1 Datia 1 Dewas 1 Dhar 4 Dindori 1 East Nimar 1 Guna 2 Gwalior 10 Harda 1 Hoshangabad 3 Indore 26 Jabalpur 5 Jhabua 2 Katni 1 Mandla 1 Mandsaur 1 Morena 1 Narsinghpur 1 Neemuch 1 Panna 1 Raisen 2 Rajgarh 2 Ratlam 2 Rewa 1 Sagar 3 Satna 2 Sehore 1 Seoni 1 Shahdol 1 Shajapur 2 Sheopur 1 Shivpuri 2 Sidhi 2 Singrauli 2 Tikamgarh 1 Ujjain 5 Vidisha 2 West Nimar 4 Bandhan Bank Ltd. -

CEO Madhya Pradesh
General Elections to Lok-Sabha -2019 (Madhya Pradesh) Parliamentary Assembly Returning Offricer ARO DEO Constituency Constituency District Name Division Name No. Name Name E-Mail Contact No. No. Name Officer Name E-Mail Contact No. Officer Name E-Mail Contact No. Shri. Devendra devendrasingh52 1Morena 1Sheopur 9806126292 SHEOPUR CHAMBAL Pratap singh [email protected] Shri Basant dmsheopur@m 9425064030 kurre p.nic.in erovjrsheopur@g 1 Morena 2 Vijaypur Shri Saurabh Mishra 8959575348 SHEOPUR CHAMBAL mail.com sdmsabalgarh@g 1 Morena 3 Sabalgarh Mr. Mrinal Meena 9111466449 MORENA CHAMBAL mail.com sdmjoura20@gm 1MorenaMs. Priyanka dmmorena 4 Joure Mr. Vinod Singh 9425338594 MORENA CHAMBAL 7898332844 ail.com Das @nic.in prakashkasbe59 1 Morena 5 Sumawali Mr. Prakash Kasbe 9425038737 MORENA CHAMBAL @gmail.com Ms. Priyanka dmmorena@ni 7898332844 suresh.jadav4@g Das c.in 1 Morena 6 Morena Mr. Suresh Jadhav 9893504461 MORENA CHAMBAL mail.com Shri. Suresh Kumar dimini07morena 1Morena 7Dimani 9926044085 MORENA CHAMBAL Barahdiya @gmail.com sdmambah123@ 1 Morena 8 Ambah (SC)Mr. Neeraj Sharma 9826248644 MORENA CHAMBAL gmail.com [email protected] 2 Bhind (SC) 9 Ater Shri Siddharth Patel 9754846815 BHIND CHAMBAL om ro.bhind1@gmail 2 Bhind (SC) 10 Bhind Shri H.B.Sharma 9425743666 BHIND CHAMBAL .com Shri Iqbal ro.lahar1@gmail. Dr vijay [email protected] 2Bhind (SC) 11Lahar 9893708227 BHIND 8435333095 CHAMBAL Mohammad com Kumar J. n ro.mehgaon@gm 2 Bhind (SC) 12 Mehgaon Shri M. K. Sharma 9424314844 BHIND CHAMBAL Shri Dr. vijay dmbhind@n ail.com 8435333095 Kumar J. ic.in ro.gohad@gmail. 2 Bhind (SC) 13 Gohad (SC)Shri D.K. -

Download Article
International Journal of Information Research and Review, January, 2020 International Journal of Information Research and Review Vol. 07, Issue, 01, pp.6668-6674, January, 2020 RESEARCH ARTICLE STUDY OF GROUND WATER QUALITY OF MANDIDEEP INDUSTRIAL AREA, MADHYA PRADESH, INDIA Reeta Kori, Alok Saxena, Harish Wankhade, Asad Baig, Ankita Kulshreshtha*, Saket Mishra and Smriti Sen Central Laboratory, Madhya Pradesh Pollution Control Board, Paryavaran Parisar, E-5, Arera Colony, Bhopal, India ARTICLE INFO ABSTRACT Article History: Ground water quality, especially within industrial area is increasing interest for study. This paper is th focus on the ground water quality status of Mandideep industrial area of Madhya Pradesh India. Study Received 10 October, 2019 of physico-chemical parameters of ground water was carried out during different quarters of year Received in revised form 07th November, 2019 2018- 2019. In present study, total thirteen ground water samples were collected from selected Accepted 29th December, 2019 locations at Mandideep industrial area. Ground water was monitored and samples were analyzed by Published online 30th January, 2020 standard methods. It is concluded that trace amount of pollutant in ground water was observed at few locations in Mandideep industrial area. The results are compared with BIS: 10500 (2012). Parameters Keywords: like fluoride and ammonical nitrogen were not detected in ground water at all monitoring locations Mandideep Industrial area, during this study. Anthropogenic activities (mainly industrial), in the area may have direct or indirect Ground Water, impact on the groundwater quality. Water Pollutants, Water quality. Copyright © 2020, Reeta Kori et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricte d use, distribution and reproduction in any medium, provided the original work is properly cited. -

Raisen District, Madhya Pradesh Field Visit Report
Raisen District, Madhya Pradesh Field Visit Report By: Dr. Arpana Kullu, Consultant NRHM-I MoHFW Introduction For the Action based Monitoring of High Focused Districts, first visit was made to the district of Raisen in Madhya Pradesh from 26th April 2010 to 30th April 2010. The facilities visited and key persons visited for monitoring are enumerated in the Table 1 shown below. The monitoring visit, out of 7 blocks, visit was made to 4 blocks and it included interaction with the Health staff including the Medical Officers, Block Programme Managers , Nursing Staff and few ASHA’s , to gain a better understanding of the processes and difficulties in functioning. DATE DISTRICT/BLOCK PLACE VISITED PERSONS VISITED 27.04.10 Raisen SPMU, Bhopal SPM- Mr. Kumar Sourav District Health Office, ASO- Mr. Raikward Raisen IEC Consultant- Mr.Amit Sharma 28.04.10 1).Obdullaganj CHC Mandideep & Block BMO- Dr.K.P.Yadav 2).Bareilly PHC(Obdullaganj) BPM- Mr.Sunil & CHC Bareilly Mr.Soni BMO- Dr.B.D.Khare 29.04.10 Raisen (Sanchi) CHC Sanchi & PHC BMO- Dr.Das Salamatpur BPM- Ms. Rashmi District Hospital MO 30.04.10 Silwani CHC Silwani BMO-Dr. Manre SC Itkhedi BPM- Deepak Singh LHV- Sulochana Table 1: Showing the Blocks and Persons visited for monitoring. Methodology Secondary Data was collected for the structured format from the state and district HMIS data format that was already available at the respective Programme Management Unit. The primary data was collected for the qualitative responses in the format through interactions with the health staff during the visits to the health facilities. -
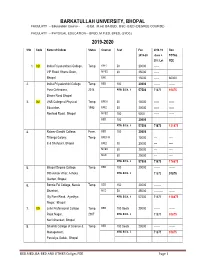
Barkatullah University, Bhopal 2019-2020
BARKATULLAH UNIVERSITY, BHOPAL FACULATY -- Eduacation Course - -B.Ed. M.ed. BA-BED., BSC.-B.ED (DEGREE COURSE) FACULATY -- PHYSICAL EDUCATION -- BPED. M.P.ED. BPES, (3YDC) 2019-2020 SNo Code Name of College Status Courses Seat Fee 2018 -19 Req. 2019-20 dues + TOTAL 25% Let FEE 1. 023 Indira Priya darshani College, Temp. BPED 50 20000 ------ VIP Road Khanu Goan, MPED 30 25000 ------ Bhopal BPE 15000 ------ 60000 2. Indira Priyadarshini College Temp. BED 100 20000 ------- ------- Pura Chhindwra, 2014 Affili. B.Ed. - ii 57500 71875 91875 Biaora Road Bhopal 3. 063 VNS College of Physical Temp. BPE III 50 15000 ------ ------ Education, 1995 BPED 30 20000 ------ ------ Neelbad Road, Bhopal MPED 100 5000 ------ ------ BED 100 20000 Affili. B.Ed. - ii 57500 71875 131875 4. Rajeev Gandhi College, Perm. BED 100 20000 Trilanga Colony, Temp. BPES III 15000 ---- ---- E-8 Shahpura, Bhopal BPED 70 20000 ---- ---- MPED 30 25000 ---- ---- MED 50 25000 ---- ---- Affili. B.Ed. - ii 57500 71875 176875 5. Bhopal Degree College, Temp. BED 100 20000 ------- ------- 393 Ashok Vihar, Ashoka Affili. B.Ed. - ii 71875 91875 Garden, Bhopal 6. Bonnie Foi College , Narela Temp. BED 150 20000 -------- Shankari, MED 50 25000 ---------- ------- By Pass Road, Ayodhya Affili. B.Ed. - ii 57500 71875 116875 Nagar, Bhopal 7. 028 Johri Prof essional College Temp. BED 100 Seats 20000 ------- ------- Rajat Nagar, 2007 Affili. B.Ed. - ii 71875 91875 Narli Shankari, Bhopal 8. Shashib College of Science & Temp. BED 100 Seats 20000 ------- ------- Management, Affili. B.Ed. - ii 71875 91875 Parvaliya Sadak, Bhopal BED.MED. BA-BED AND OTHER Collges FEE Page 1 9. Takshila College, Jhirniya 2004 BED 100 Seats 20000 ------- ------- Biaora Road, NH-12,Bhopal Temp. -

Industrial Land Bank 2016
22 - 23 2016 22 - 23 2016 INDUSTRIAL LAND BANK 2016 22 - 23 2016 INDUSTRIAL LAND BANK 2016 22 - 23 2016 22 - 23 2016 Publisher: MP Trade and Investment Facilitation Corporation Limited “CEDMAP BHAWAN” 16-A, Arera Hills Bhopal - 462001, M.P(India) Tel. :(91) 755-2575618, 2571830 Fax : (91) 755-2559973 E-mail : [email protected] http://www.mptrifac.gov.in http://www.invest.mp.gov.in/ INDUSTRIAL LAND BANK 2016 CONTENT 1. Preface 2 2. Introduction 3 3. Industrial land bank available with Government of Madhya Pradesh 5 4. Industrial land bank with Audyogik Kendra Vikas Nigam (AKVN) 6 4a. Developed/Developing land bank available with AKVNs 9 • Bhopal AKVN 9 • IIDC Gwalior 22 • Indore AKVN 36 • Jabalpur AKVN 70 • Rewa AKVN 81 • Sagar AKVN 89 • Ujjain AKVN 99 4b. Undeveloped land bank available with AKVNs 113 4c. Undeveloped land allotment guidelines 131 5. Developed Industrial land bank with department of Micro Small and Medium Enterprises (MSME) 137 6. Industrial land bank available with Madhya Pradesh State Electronic Development Corporation (MPSEDC) 143 1 INDUSTRIAL LAND BANK 2016 1 2 INDUSTRIAL LAND BANK 2016 2 INTRODUCTION Madhya Pradesh has emerged as a Growth Centre of the country in the last decade. Madhya Pradesh has witnessed a radical transformation in terms of economic and social development. Madhya Pradesh’s central location makes it an ideal destination as a manufacturing and sourcing hub to tap the constantly growing Indian market. Owing to its rich soil, several rivers and large irrigation projects, Madhya Pradesh is a thriving destination for agriculture and food processing business. -

ICAR-Indian Institute of Soil Science Nabibagh, Berasia Road, Bhopal-462 038, India
ICAR-Indian Institute of Soil Science Nabibagh, Berasia road, Bhopal-462 038, India Report of the ICAR short course on “Advances in Assessment of Soil Pollution and its Remediation” The ICAR-IISS, Bhopal conducted a ICAR Short course on “Advances in Assessment of Soil Pollution and its Remediation” during 16-25th March, 2017. The training was inaugurated on 16th March morning by Dr A K Patra, Director, ICAR-IISS, Bhopal in the presence of participants, IISS scientific staff and training related persons. The Course director Dr J K Saha briefly elaborated the need of this course in present era and how to tackle to reduce or manage the adverse effects of environmental pollution with advanced techniques. All the resource persons delivered their assigned talk in a very effective way that has been reflected by participants’ feedback on the training. In this Short course, participants were exposed to modern instruments like ICP-OES, FT-IR, IR spectroscopy, GC, HPLC, TOC, Lypholyser etc through several practical classes and were properly explained/trained on the precautions and maintenance of the instruments. The feedback revealed that majority of the participants found the training most useful and lectures were of high quality. There has been overall improvement in knowledge level of participants on the subject as indicated by average score of 86.5% in evaluation test conducted at the end of the Short course as against the average score level 48.3% in evaluation test at the beginning. The boarding and lodging, laboratory facilities, resource persons and their way of teaching, behavior, decision making and selected topics were highly appreciated by the participants. -

Madhya Pradesh Size:( 5.5
37th Meeting of the Central Sanctioning cum Monitoring Committee(CSMC) under Pradhan Mantri Awas Yojana - Housing For All rd Urban Development & Housing Department 23 August, 2018 Government of Madhya Pradesh Indicators Current Status (No.) . Cities Approved 378 . Demand Survey Completed 378 . Total Demand 11.52 Lakh . Demand received through Common Service Centre 4,44,606 and Online Application . Cases accepted/rejected 2,07,397 . Whether HFAPoA Submitted Yes, For all 378 Towns . Whether AIP Submitted Yes 10,49,665 Surveyed Data Entries have been entered in PMAY . Whether HFAPoA & AIP entered in MIS MIS . SLTC/CLTC staffs approved vs. placed SLTC:10 vs 10 / CLTC: 454 vs 521 Sanctioning: 2.47 Lakh DUs (Excluding CLSS) . Target of DUs in 2018-19 Completion: 5.00 Lakh DUs As per provision of GoI matching budgetary provisions is . State Budgetary Provision for PMAY (U) in 2018-19 ensured in state budget 2 Indicators Current Status (No.) .Survey entry made (%) 87.60% .Projects approved: 887 .Projects entered (7A/B/C/D) 851 .DUs approved under BLC 3,47,242 (Excluding 35,475 Surrendered DUs) .Beneficiaries attached 3,04,186 .Geo-tagged Points 6,77,539 (No. of Unique Houses Geo-Tagged: 2,56,075) 3 Grounded for Construction / In-Progress EWS Work Verticals Houses Tendered Order Completed Approved Issued Foundation Lintel Roof Total AHP 1,49,645 48,499 1,01,146 58,816 18,643 5,748 83,207 17,939 (Including RAY) BLC (N) 3,47,242 - - 1,24,110 26,775 18,767 1,69,652 97,313 ISSR 2,172 960 - - - - - - CLSS 11,616 - - - - - - 11,616 (Including LIG/MIG) -

30. Milk Chilling Infrastructure Expansion in District Sehore
Success Story of Milk Chilling infrastructure expansion in district Sehore under Rastriya Krishi Vikas Yojana. Background Dairying was hardly known as an organized activity at the time of formation of the State of Madhya Pradesh in 1956. The dairy trade was unorganized and largely under the control of milk traders, middlemen and vendors. Dairying, as an allied activity to agriculture, provides many rural families with their only source of continuous income. Lately, the concept has undergone a lot of change owing to the participative and interactive approach having been organized on proper lines as an integrated and inter-linked activity involving production, processing and marketing. Bhopal Sahakari Dugdh Sangh Maryadit, Bhopal a regional cooperative milk producers' entity, affiliated to MP State Cooperative Dairy Federation Ltd., Bhopal, was established in the year 1976. The milk union was aimed at establishing dairying network and related processing & chilling infrastructure in the ambit of Bhopal milkshed thus covering 11 districts, 68 Tehsils and Blocks. Since then, the milk union has developed manifold and currently has the spread of following in its fold - 1. Fluid Milk Dairy plant of 1.50 Lakh Litres Per day capacity, 2. Cattle Feed plant of 100 Metric Ton per day manufacture capacity 3. 16 milk Chilling Centers in the milkshed area. In addition, currently (in November 2011) the Bhopal milk union has 1,263 functional Dairy Cooperative Societies collecting an average of 2,36,273 liters daily from its 62,328 milk producer members. The member classification represents 22% General, 68% OBC, 8% Scheduled Caste and 2% Scheduled Tribe category. -

Prepared By, ABC TECHNO LABS INDIA PVT.LTD
Executive Summary For Proposed Integrated Municipal Solid Waste Management Plant (including 23 MW power plant) at Adampur Chhavani, Phanda Block, Huzur Tehsil, Bhopal District, Madhya Pradesh. Prepared By, ABC TECHNO LABS INDIA PVT.LTD. AN ISO ISO 9001:2008, ISO14001:2004 & OHSAS 18001:2007 certified Environmental Engineering and Consultancy Organization (NABL Accredited & MoEF Recognised Environment Laboratory) QCI NABET Accredited (Certificate No. NABET / EIA / 1316 / RA001) Corporate Office: No.2, 2nd Street, Thangam Colony, Anna Nagar West, Chennai – 600040. Tamil Nadu, India. Tel: 044 – 26161123 / 24 / 25 Mumbai Office: A-355, Balaji Bhavan, Plot No. 42 A, Sector 11, CBD Belapur, Navi Mumbai – 400614. Maharashtra, India Tel: 022 27580044 www.abctechnolab.com [email protected] EXECUTIVE SUMMARY NAME OF THE PROJECT: Environmental Clearance for proposed Integrated Municipal Solid Waste Management Plant at Adampur Chhavani, Phanda Block, Huzur Tehsil, Bhopal District by M/s Bhopal Municipal Solid Waste Private Limited. LOCATION: Adampur Chhavani, Phanda Block, Huzur Tehsil, Bhopal District, Madhya Pradesh. The proposed project land is of Bhopal Municipal Corporation (BMC) and it does not involve any forest land. The Geographical co-ordinates of the project site are: Corner No. Latitude (DD MM SS.s) Longitude (DD MM SS.s) 1 23 Degree 15’59.5’’N 77 Degree 33’21.6’’E 2 23 Degree 15’59.4’’N 77 Degree 33’44.5’’E 3 23 Degree 15’50.3’’N 77 Degree 33’44.7’’E 4 23 Degree 15’50.2’’N 77 Degree 33’21.3’’E (The project study area of 10 KM radius around the project site does not involve any Reserved Protected Forest areas, Wild Life Sanctuary, Hills/Valleys, Defense Installations).